25.6 Physiology of Urine Formation: Medullary Concentration Gradient
Learning Objectives
- Explain the role of the loop of Henle, the vasa recta, and the countercurrent multiplication mechanisms in urine production
- Explain the role of aldosterone and ADH in urine production
Countercurrent Multiplier System
The structure of the loop of Henle and associated peritubular capillary create a countercurrent multiplier system (Figure 25.6.1). The countercurrent term comes from the fact that the descending and ascending loops are next to each other and their fluid flows in opposite directions (countercurrent). The multiplier term is due to the action of solute pumps that increase (multiply) the concentrations of urea and Na+ deep in the medulla, as described next.
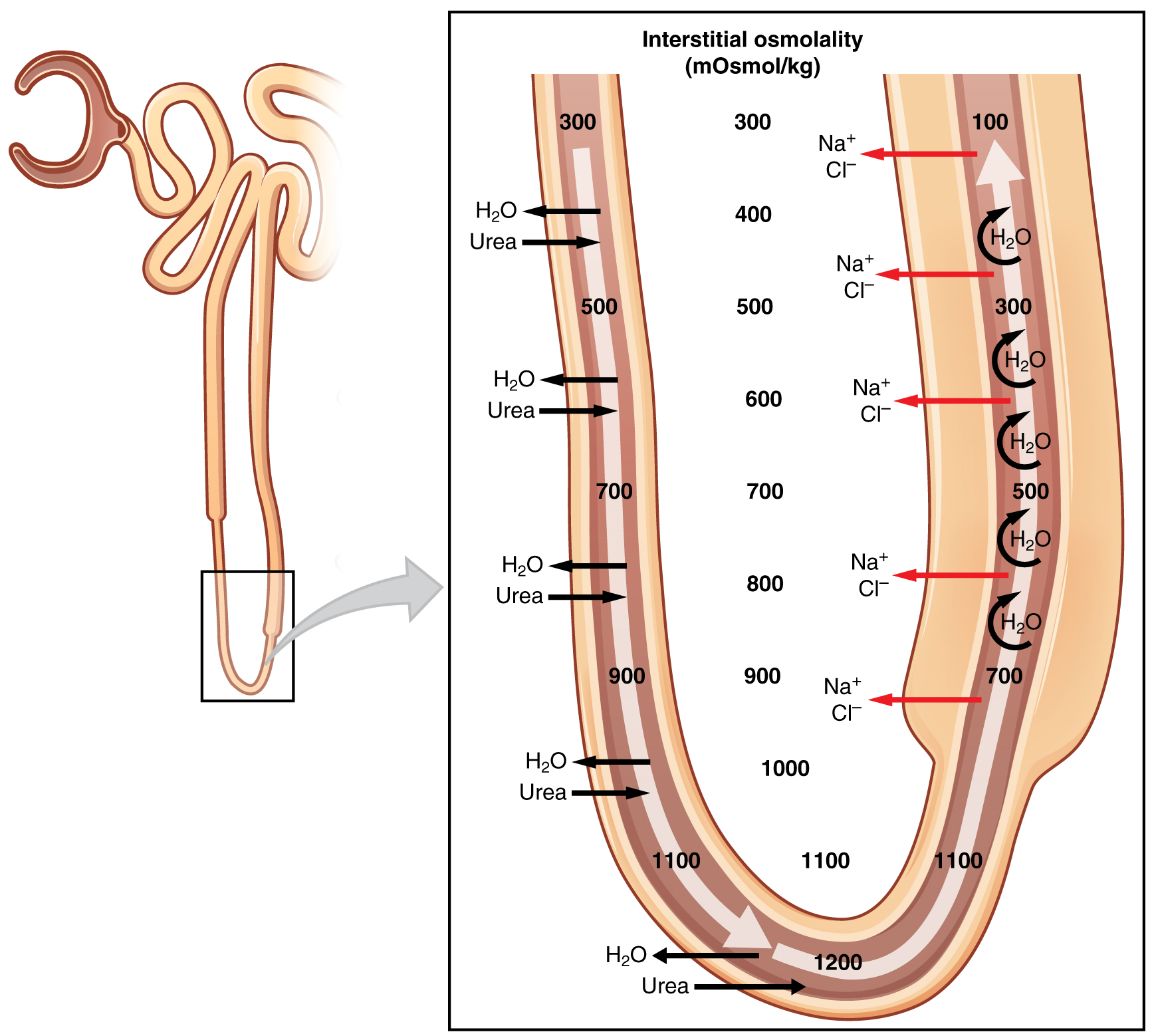
The presence of aquaporin channels in the descending loop allows prodigious quantities of water to leave the loop and enter the hyperosmolar interstitium of the pyramid, where it is returned to the circulation by the vasa recta. As the loop turns to become the ascending loop, there is an absence of aquaporin channels, so water cannot leave the loop. However, in the basal membrane of cells of the thick ascending loop, ATPase pumps actively remove Na+ from the cell into the interstitial space. A Na+/K+/2Cl– symporter in the apical membrane passively allows these ions to enter the cell cytoplasm from the lumen of the loop down a concentration gradient created by the pump. This mechanism works to dilute the fluid of the ascending loop ultimately to approximately 50–100 mOsmol/L.
At the same time that water is freely diffusing out of the descending loop through aquaporin channels into the interstitial spaces of the medulla, urea freely diffuses into the lumen of the descending loop as it descends deeper into the medulla, much of it to be reabsorbed from the filtrate when it reaches the collecting duct. In addition, collecting ducts have urea pumps that actively pump urea into the interstitial spaces. This results in the recovery of Na+ to the circulation via the vasa recta and creates a high osmolar environment in the depths of the medulla. Thus, the movement of Na+ and urea into the interstitial spaces by these mechanisms creates the hyperosmotic environment in the depths of the medulla. The net result of this countercurrent multiplier system is to recover both water and Na+ in the circulation.
At the transition from the DCT to the collecting duct, about 20 percent of the original water is still present and about 10 percent of the sodium. If no other mechanism for water reabsorption existed, about 20–25 liters of urine would be produced. Now consider what is happening in the adjacent capillaries, the vasa recta. They are recovering both solutes and water at a rate that preserves the countercurrent multiplier system. In general, blood flows slowly in capillaries to allow time for exchange of nutrients and wastes. In the vasa recta particularly, this rate of flow is important for two additional reasons. The flow must be slow to allow blood cells to lose and regain water without either crenating or bursting. Second, a rapid flow would remove too much Na+ and urea, destroying the osmolar gradient that is necessary for the recovery of solutes and water. Thus, by flowing slowly to preserve the countercurrent mechanism, as the vasa recta descend, Na+ and urea are freely able to enter the capillary, while water freely leaves; as they ascend, Na+ and urea are secreted into the surrounding medulla, while water reenters and is removed.
Hormonal Influence on Reabsorption of Water
The renal medulla has a concentration gradient with a low osmolarity superficially and a high osmolarity at its deepest point. The kidneys have expended a large amount of cellular energy to create this gradient, but what do the nephrons do with this gradient? In the presence of hormones, the kidney is able to concentrate the filtrate to be 20 times more concentrated than the glomerular plasma and PCT filtrate.
The process of concentrating the filtrate occurs in the DCT and collecting ducts. Recall that the DCT and collecting ducts are lined with simple cuboidal epithelium with receptors for aldosterone and ADH, respectively. Solutes move across the membranes of the cells of the DCT and collecting ducts, which contain two distinct cell types, principal cells and intercalated cells. A principal cell possesses channels for the recovery or loss of sodium and potassium. An intercalated cell secretes or absorbs acid or bicarbonate. As in other portions of the nephron, there is an array of micromachines (pumps and channels) on display in the membranes of these cells.
Regulation of urine volume and osmolarity are major functions of the collecting ducts. By varying the amount of water that is recovered, the collecting ducts play a major role in maintaining the body’s normal osmolarity. If the blood becomes hyperosmotic, the collecting ducts recover more water to dilute the blood; if the blood becomes hyposmotic, the collecting ducts recover less of the water, leading to concentration of the blood. Another way of saying this is: If plasma osmolarity rises, more water is recovered and urine volume decreases; if plasma osmolarity decreases, less water is recovered and urine volume increases. This function is regulated by the posterior pituitary hormone ADH (vasopressin). With mild dehydration, plasma osmolarity rises slightly. This increase is detected by osmoreceptors in the hypothalamus, which stimulates the release of ADH from the posterior pituitary. If plasma osmolarity decreases slightly, the opposite occurs.
When stimulated by ADH, the principal cells of the collecting duct will insert aquaporin channels proteins into their apical membranes. Recall that aquaporins allow water to pass from the duct lumen across the lipid-rich, hydrophobic cell membranes to travel through the cells and into the interstitial spaces where the water will be recovered by the vasa recta. As the ducts descend through the medulla, the osmolarity surrounding them increases (due to the countercurrent mechanisms described above). If aquaporin water channels are present, water will be osmotically pulled from the collecting duct into the surrounding interstitial space and into the peritubular capillaries. This process allows for the recovery of large amounts of water from the filtrate back into the blood, which produces a more concentrated urine. If less ADH is secreted, fewer aquaporin channels are inserted and less water is recovered, resulting in dilute urine. By altering the number of aquaporin channels, the volume of water recovered or lost is altered. This, in turn, regulates the blood osmolarity, blood pressure, and osmolarity of the urine.
As Na+ is pumped from the filtrate, water is passively recaptured for the circulation; this preservation of vascular volume is critically important for the maintenance of a normal blood pressure. Aldosterone is secreted by the adrenal cortex in response to angiotensin II stimulation. As an extremely potent vasoconstrictor, angiotensin II functions immediately to increase blood pressure. By also stimulating aldosterone production, it provides a longer-lasting mechanism to support blood pressure by maintaining vascular volume (water recovery).
In addition to receptors for ADH, principal cells have receptors for the steroid hormone aldosterone. While ADH is primarily involved in the regulation of water recovery, aldosterone regulates Na+ recovery. Aldosterone stimulates principal cells to manufacture luminal Na+ and K+ channels as well as Na+/K+ ATPase pumps on the basal membrane of the cells of the DCT and collecting duct. When aldosterone output increases, more Na+ is recovered from the filtrate and water follows the Na+ passively. The movement of Na+ out of the lumen of the collecting duct creates a negative charge that promotes the movement of Cl– out of the lumen into the interstitial space by a paracellular route across tight junctions. Peritubular capillaries (or vasa recta) receive the solutes and water, returning them to the circulation. As the pump recovers Na+ for the body, it is also pumping K+ into the filtrate, since the pump moves K+ in the opposite direction.
Chapter Review
The kidney regulates water recovery and blood pressure by producing the enzyme renin. It is renin that starts a series of reactions, leading to the production of the vasoconstrictor angiotensin II and the salt-retaining steroid aldosterone. Water recovery is also powerfully and directly influenced by the hormone ADH. Even so, it only influences the last 10 percent of water available for recovery after filtration at the glomerulus, because 90 percent of water is recovered before reaching the collecting ducts. Depending on the body’s fluid status at any given time, the collecting ducts can recover none or almost all of the water reaching them.
The descending and ascending limbs of the loop of Henle consist of thick and thin segments. Absorption and secretion continue in the DCT but to a lesser extent than in the PCT. Each collecting duct collects forming urine from several nephrons and responds to the posterior pituitary hormone ADH by inserting aquaporin water channels into the cell membrane to fine tune water recovery.
The ascending loop is impervious to water but actively recovers Na+, reducing filtrate osmolarity to 50–100 mOsmol/kg. The descending and ascending loop and vasa recta form a countercurrent multiplier system to increase Na+ concentration in the kidney medulla. The collecting ducts actively pump urea into the medulla, further contributing to the high osmotic environment. The vasa recta recover the solute and water in the medulla, returning them to the circulation. Nearly 90 percent of water is recovered before the forming urine reaches the DCT, which will recover another 10 percent.
Review Questions
1. Aquaporin channels are only found in the collecting duct.
- true
- false
3. The fine tuning of water recovery or disposal occurs in ________.
- the proximal convoluted tubule
- the collecting ducts
- the ascending loop of Henle
- the distal convoluted tubule
Critical Thinking Questions
1. Which vessels and what part of the nephron are involved in countercurrent multiplication?
2. Give the approximate osmolarity of fluid in the proximal convoluted tubule, deepest part of the loop of Henle, distal convoluted tubule, and the collecting ducts.
Glossary
- countercurrent multiplier system
- involves the descending and ascending loops of Henle directing forming urine in opposing directions to create a concentration gradient when combined with variable permeability and sodium pumping
- intercalated cell
- specialized cell of the collecting ducts that secrete or absorb acid or bicarbonate; important in acid–base balance
- leaky tight junctions
- tight junctions in which the sealing strands of proteins between the membranes of adjacent cells are fewer in number and incomplete; allows limited intercellular movement of solvent and solutes
- principal cell
- found in collecting ducts and possess channels for the recovery or loss of sodium and potassium; under the control of aldosterone; also have aquaporin channels under ADH control to regulate recovery of water
Solutions
Answers for Review Questions
- B
- B
Answers for Critical Thinking Questions
- The vasa recta and loop of Henle are involved in countercurrent multiplication.
- The approximate osmolarities are: CT = 300; deepest loop = 1200; DCT = 100; and collecting ducts = 100–1200.
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax, are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.


