19.1 Heart Anatomy
Learning Objectives
By the end of this section, you will be able to:
- Describe the size and location of the heart within the thoracic cavity
- Distinguish between the systemic and pulmonary circulations including the pathway of oxygenated and deoxygenated blood
- Describe the coverings, the surface anatomy and tissue layers of the heart
- Describe the internal anatomy of the heart including the chambers and valves and relate the internal features to how it functions as a pump
- Trace the coronary circulation and explain why this ciruclation is needed
The vital importance of the heart is obvious. If one assumes an average rate of contraction of 75 contractions per minute, a human heart would contract approximately 108,000 times in one day, more than 39 million times in one year, and nearly 3 billion times during a 75-year lifespan. Each of the major pumping chambers of the heart ejects approximately 70 mL blood per contraction in a resting adult. This would be equal to 5.25 liters of fluid per minute and approximately 14,000 liters per day. Over one year, that would equal 10,000,000 liters or 2.6 million gallons of blood sent through roughly 60,000 miles of vessels. In order to understand how that happens, it is necessary to understand the anatomy and physiology of the heart.
Location and Size of the Heart
The human heart is located within the thoracic cavity, medially between the lungs in the space known as the mediastinum. Figure 19.1.1 shows the position of the heart within the thoracic cavity. Within the mediastinum, the heart is separated from the other mediastinal structures by a tough membrane known as the pericardium, or pericardial sac, and sits in its own space called the pericardial cavity. The dorsal surface of the heart lies near the bodies of the vertebrae, and its anterior surface sits deep to the sternum and costal cartilages. The great veins, the superior and inferior venae cavae, and the great arteries, the aorta and pulmonary trunk, are attached to the superior surface of the heart, called the base. The base of the heart is located at the level of the third costal cartilage, as seen in Figure 19.1.1. The inferior tip of the heart, the apex, lies just to the left of the sternum between the junction of the fourth and fifth ribs near their articulation with the costal cartilages. The right side of the heart is deflected anteriorly, and the left side is deflected posteriorly. It is important to remember the position and orientation of the heart when placing a stethoscope on the chest of a patient and listening for heart sounds, and also when looking at images taken from a midsagittal perspective. The slight deviation of the apex to the left is reflected in a depression in the medial surface of the inferior lobe of the left lung, called the cardiac notch.
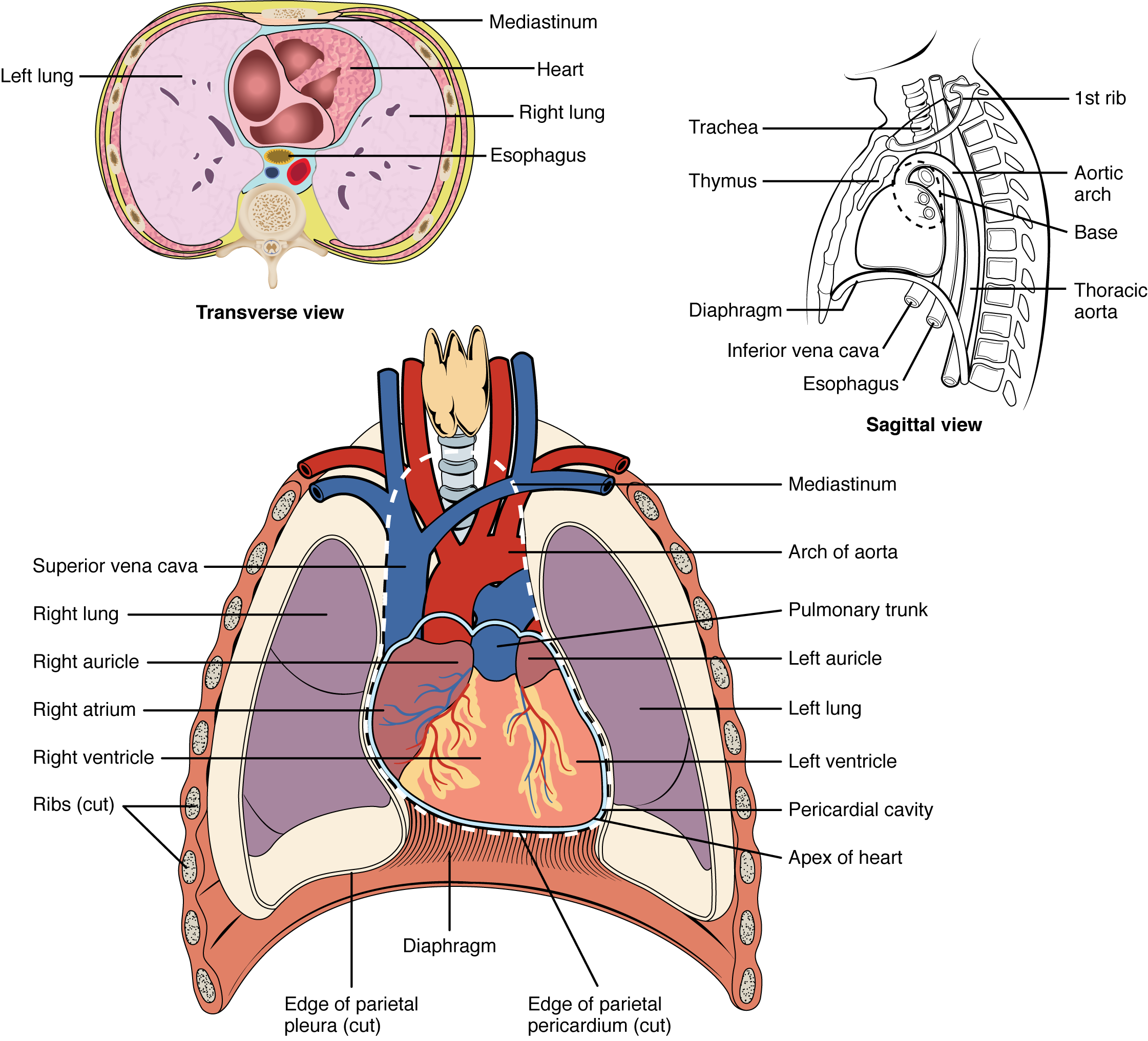
Shape and Size of the Heart
The shape of the heart is similar to a inverted pear, rather broad at the superior surface and tapering to the apex (see Figure 19.1.1). A typical heart is approximately the size of your fist: 12 cm (5 in) in length, 8 cm (3.5 in) wide, and 6 cm (2.5 in) in thickness. Given the size difference between most members of the sexes, the weight of a female heart is approximately 250–300 grams (9 to 11 ounces), and the weight of a male heart is approximately 300–350 grams (11 to 12 ounces). The heart of a well-trained athlete, especially one specializing in aerobic sports, can be considerably larger than this. Cardiac muscle responds to exercise in a manner similar to that of skeletal muscle. That is, exercise results in the addition of protein myofilaments that increase the size of the individual cells without increasing their numbers, a concept called hypertrophy. Hearts of athletes can pump blood more effectively at lower rates than those of nonathletes. Enlarged hearts are not always a result of exercise; they can result from pathologies, such as hypertrophic cardiomyopathy. The cause of such an abnormally enlarged heart muscle is unknown, but the condition is often undiagnosed and can cause sudden death in apparently otherwise healthy young people.
Circulation through the Heart and Body
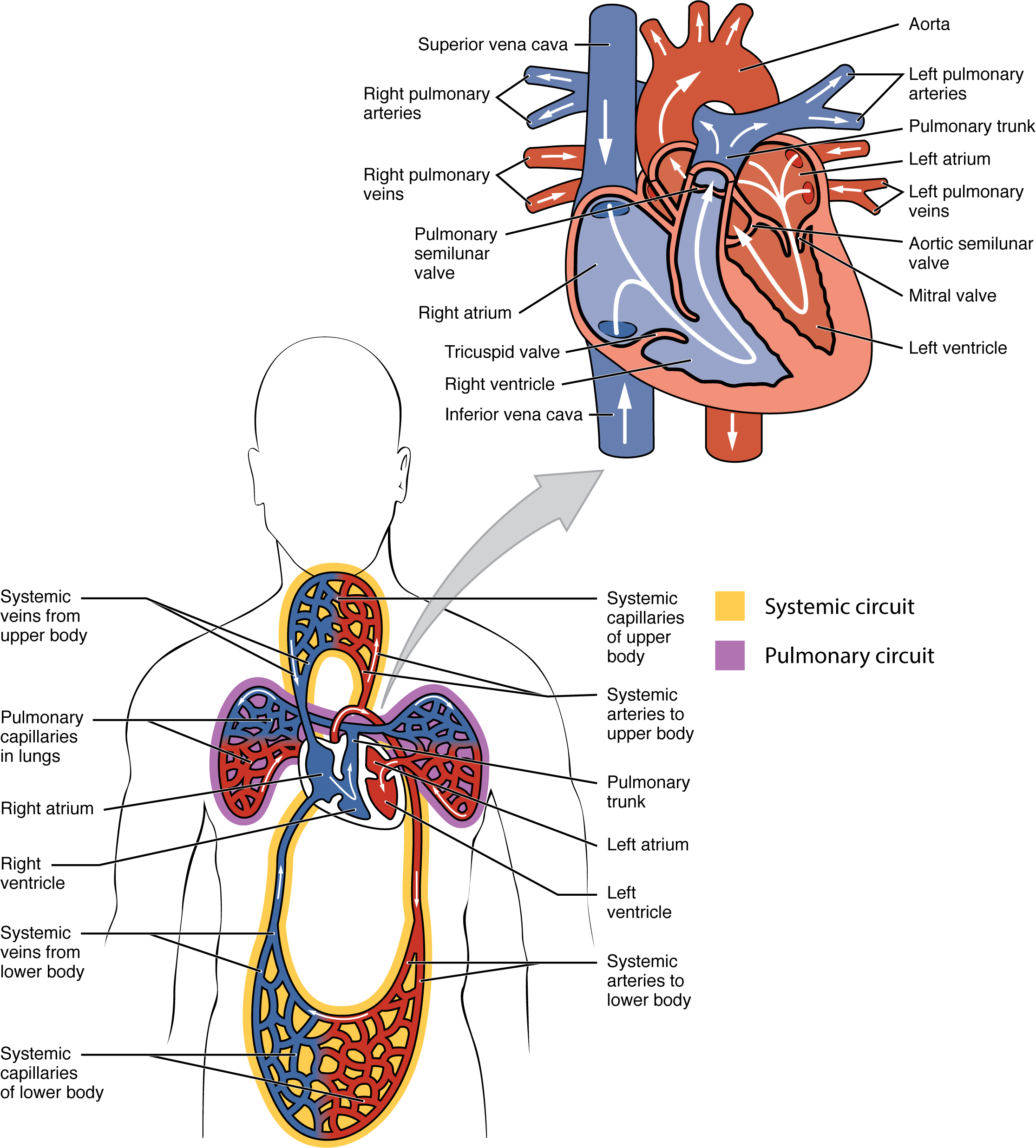
The human heart consists of four chambers: The left side and the right side each have one atrium and one ventricle. Each of the upper chambers, the right atrium (plural = atria) and the left atrium, act as receiving chambers and the combination of gravity and atrial contraction move blood into the lower chambers, the right ventricle and the left ventricle. The ventricles serve as the primary pumping chambers of the heart, propelling blood to the lungs or to the rest of the body.
There are two distinct but linked circuits in the human circulation called the pulmonary and systemic circuits. Although both circuits transport blood and everything it carries (see section 18.1), we can initially view the circuits from the point of view of gases. The pulmonary circuit transports blood to and from the lungs, where it picks up oxygen and delivers carbon dioxide for exhalation. The systemic circuit transports oxygenated blood to virtually all of the tissues of the body and returns relatively deoxygenated blood and carbon dioxide to the heart to be sent back to the pulmonary circulation. These two circulations function simultaneously and thus the heart functions as a dual pump.
The right ventricle pumps deoxygenated blood into the pulmonary trunk, which leads toward the lungs and bifurcates into the left and right pulmonary arteries. Arteries carry blood away from the heart. These vessels in turn branch many times before reaching the pulmonary capillaries, where gas exchange occurs: Carbon dioxide diffuses out of the blood and oxygen diffuses into the blood. The pulmonary trunk arteries and their branches are the only arteries in the post-natal body that carry relatively deoxygenated blood. Highly oxygenated blood returning from the pulmonary capillaries in the lungs passes through a series of vessels that join together to form the pulmonary veins—the only post-natal veins in the body that carry highly oxygenated blood. Veins carry blood toward the heart. The pulmonary veins conduct blood into the left atrium, which pumps the blood into the left ventricle, which in turn pumps oxygenated blood into the aorta and on to the many branches of the systemic circuit. Eventually, these vessels will lead to the systemic capillaries, where exchange with the tissue fluid and cells of the body occurs. In this case, oxygen and nutrients out of the systemic capillaries to be used by the cells in their metabolic processes, and carbon dioxide and waste products diffuse into the blood.
The blood exiting the systemic capillaries is lower in oxygen concentration than when it entered. The capillaries will ultimately unite to form venules, joining to form ever-larger veins, eventually flowing into the two major systemic veins, the superior vena cava and the inferior vena cava, which return blood to the right atrium. The blood in the superior and inferior venae cavae flows into the right atrium, which pumps blood into the right ventricle. This process of blood circulation continues as long as the individual remains alive. Understanding the flow of blood through the pulmonary and systemic circuits is critical to all health professions (Figure 19.1.2).
Coverings, Surface Features, and Layers
Our exploration of more in-depth heart structures begins by examining the coverings that surround the heart, the prominent surface features of the heart, and the layers that form the wall of the heart. Each of these components plays its own unique role in terms of function.
Coverings
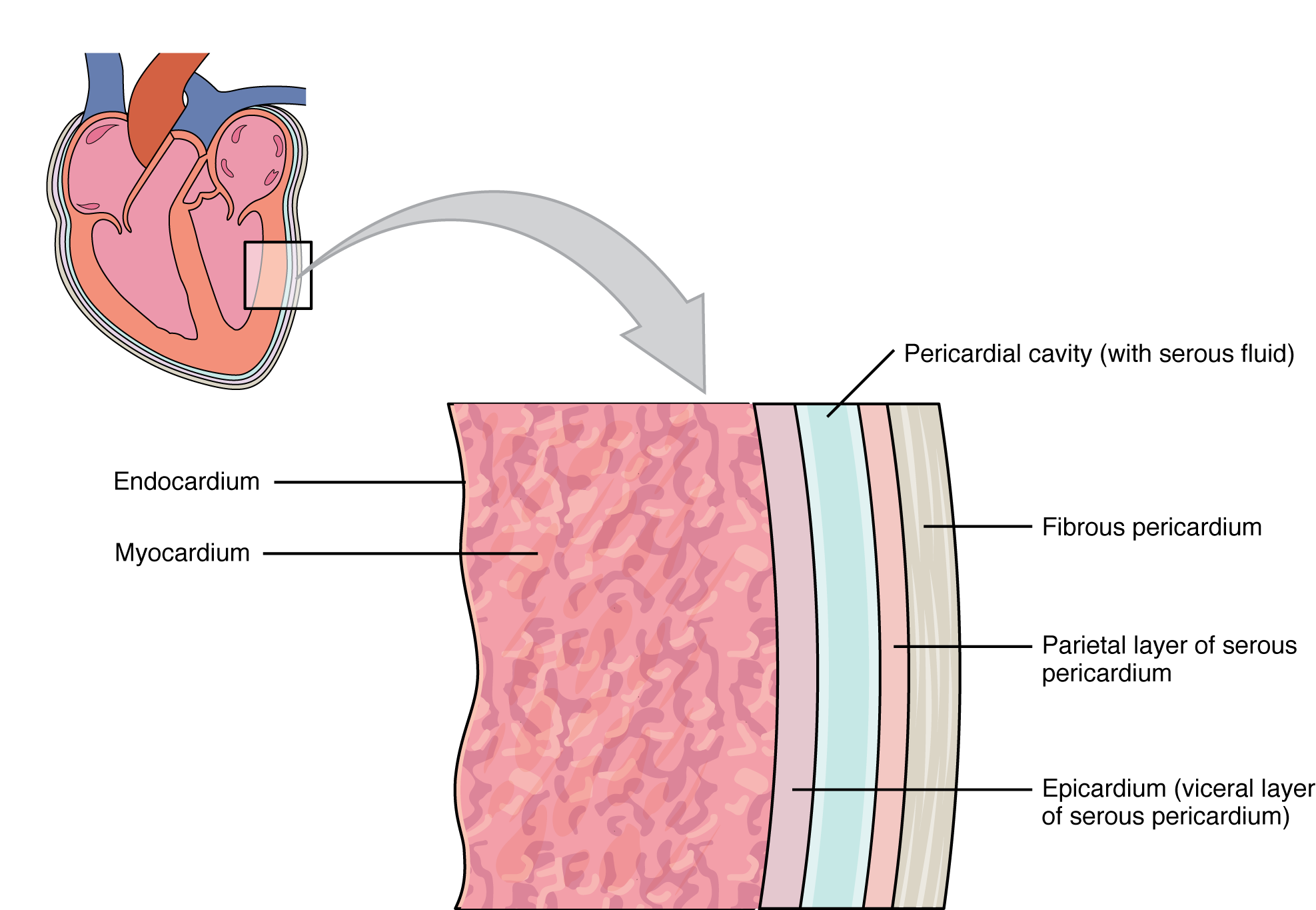
The covering that directly surrounds the heart and defines the pericardial cavity is called the pericardium or pericardial sac. It also surrounds the “roots” of the major vessels, or the areas of closest proximity to the heart. The pericardium, which literally translates as “around the heart,” consists of two distinct sublayers: the sturdy outer fibrous pericardium and the inner serous pericardium. The fibrous pericardium is made of tough, dense connective tissue that protects the heart and maintains its position in the thorax. The more delicate serous pericardium consists of two layers: the parietal pericardium, which is fused to the fibrous pericardium, and an inner visceral pericardium, or epicardium, which is fused to the heart and is part of the heart wall. The pericardial cavity, filled with lubricating serous fluid, lies between the epicardium and the pericardium.
The epicardium consists of a simple squamous epithelium called a mesothelium, reinforced with loose, irregular, or areolar connective tissue that attaches to the pericardium. This mesothelium secretes the lubricating serous fluid that fills the pericardial cavity and reduces friction as the heart contracts. Figure 19.1.3 illustrates the pericardial membrane and the layers of the heart.
Everyday Connection – CPR
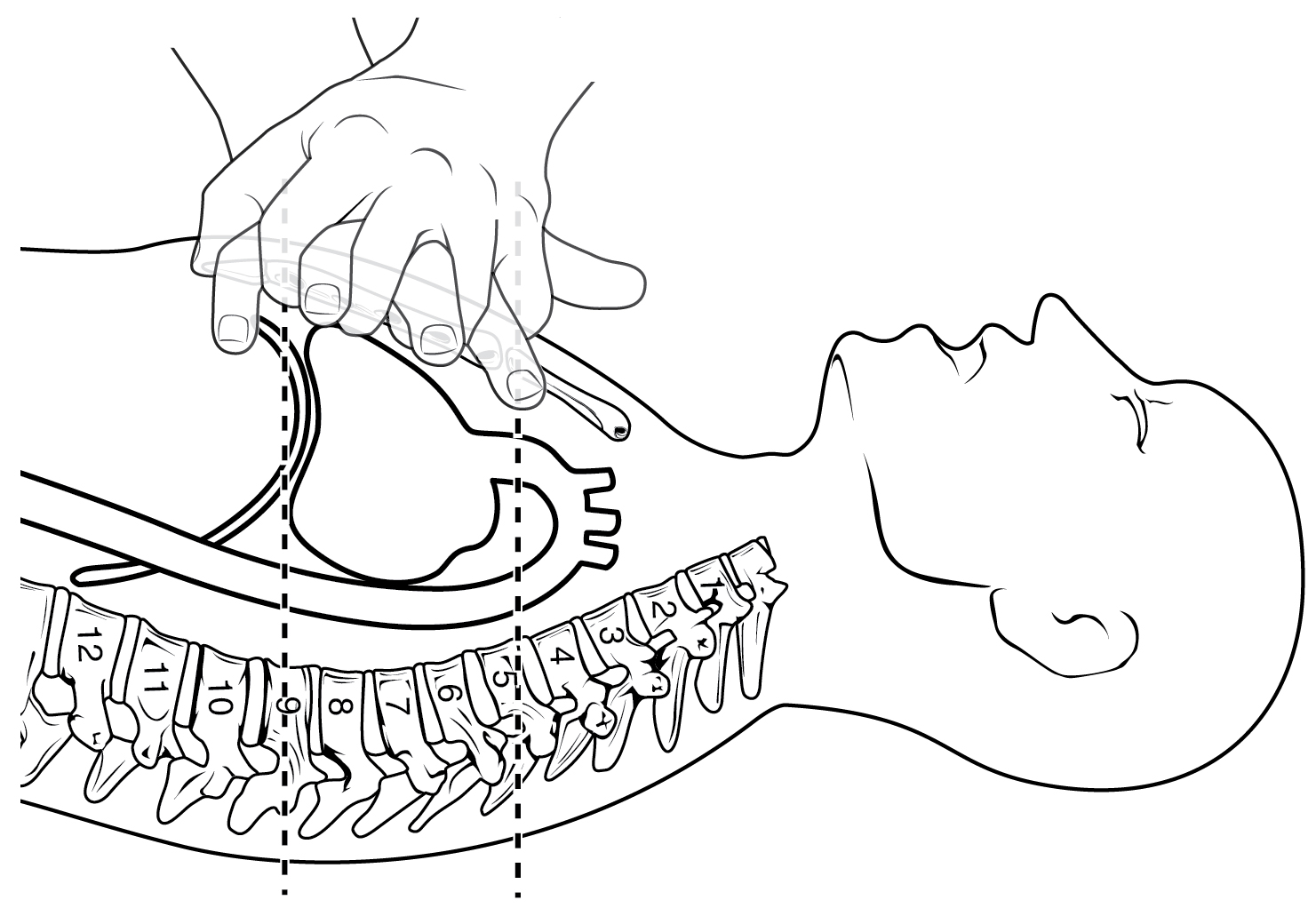
The position of the heart in the torso between the vertebrae and sternum (see Figure 19.1.1 for the position of the heart within the thorax) allows for individuals to apply an emergency technique known as cardiopulmonary resuscitation (CPR) if the heart of a patient should stop. By applying pressure with the flat portion of one hand on the sternum in the area between the line at T4 and T9 (Figure 19.1.4), it is possible to manually compress the blood within the heart enough to push some of the blood within it into the pulmonary and systemic circuits. This is particularly critical for the brain, as irreversible damage and death of neurons occur within minutes of loss of blood flow. Current standards call for compression of the chest at least 5 cm deep and at a rate of 100 compressions per minute, a rate equal to the beat in “Staying Alive,” recorded in 1977 by the Bee Gees. If you are unfamiliar with this song, a version is available on www.youtube.com. At this stage, the emphasis is on performing high-quality chest compressions, rather than providing artificial respiration. CPR is generally performed until the patient regains spontaneous contraction or is declared dead by an experienced healthcare professional.
When performed by untrained or overzealous individuals, CPR can result in broken ribs or a broken sternum, and can inflict additional severe damage on the patient. It is also possible, if the hands are placed too low on the sternum, to manually drive the xiphoid process into the liver, a consequence that may prove fatal for the patient. Proper training is essential. This proven life-sustaining technique is so valuable that virtually all medical personnel as well as concerned members of the public should be certified and routinely recertified in its application. CPR courses are offered at a variety of locations, including colleges, hospitals, the American Red Cross, and some commercial companies. They normally include practice of the compression technique on a mannequin.
External Website

Visit the American Heart Association website to help locate a course near your home in the United States. There are also many other national and regional heart associations that offer the same service, depending upon the location.
If excess fluid builds within the pericardial space, it can lead to a condition called cardiac tamponade, or pericardial tamponade. With each contraction of the heart, more fluid—in most instances, blood—accumulates within the pericardial cavity. In order to fill with blood for the next contraction, the heart must relax. However, the excess fluid in the pericardial cavity puts pressure on the heart and prevents full relaxation, so the chambers within the heart contain slightly less blood as they begin each heart cycle. Over time, less and less blood is ejected from the heart. If the fluid builds up slowly, as in hypothyroidism, the pericardial cavity may be able to expand gradually to accommodate this extra volume. Some cases of fluid in excess of one liter within the pericardial cavity have been reported. Rapid accumulation of as little as 100 mL of fluid following trauma may trigger cardiac tamponade. Other common causes include myocardial rupture, pericarditis, cancer, or even cardiac surgery. Removal of this excess fluid requires insertion of drainage tubes into the pericardial cavity. Premature removal of these drainage tubes, for example, following cardiac surgery, or clot formation within these tubes are causes of this condition. Untreated, cardiac tamponade can lead to death.
Surface Features of the Heart
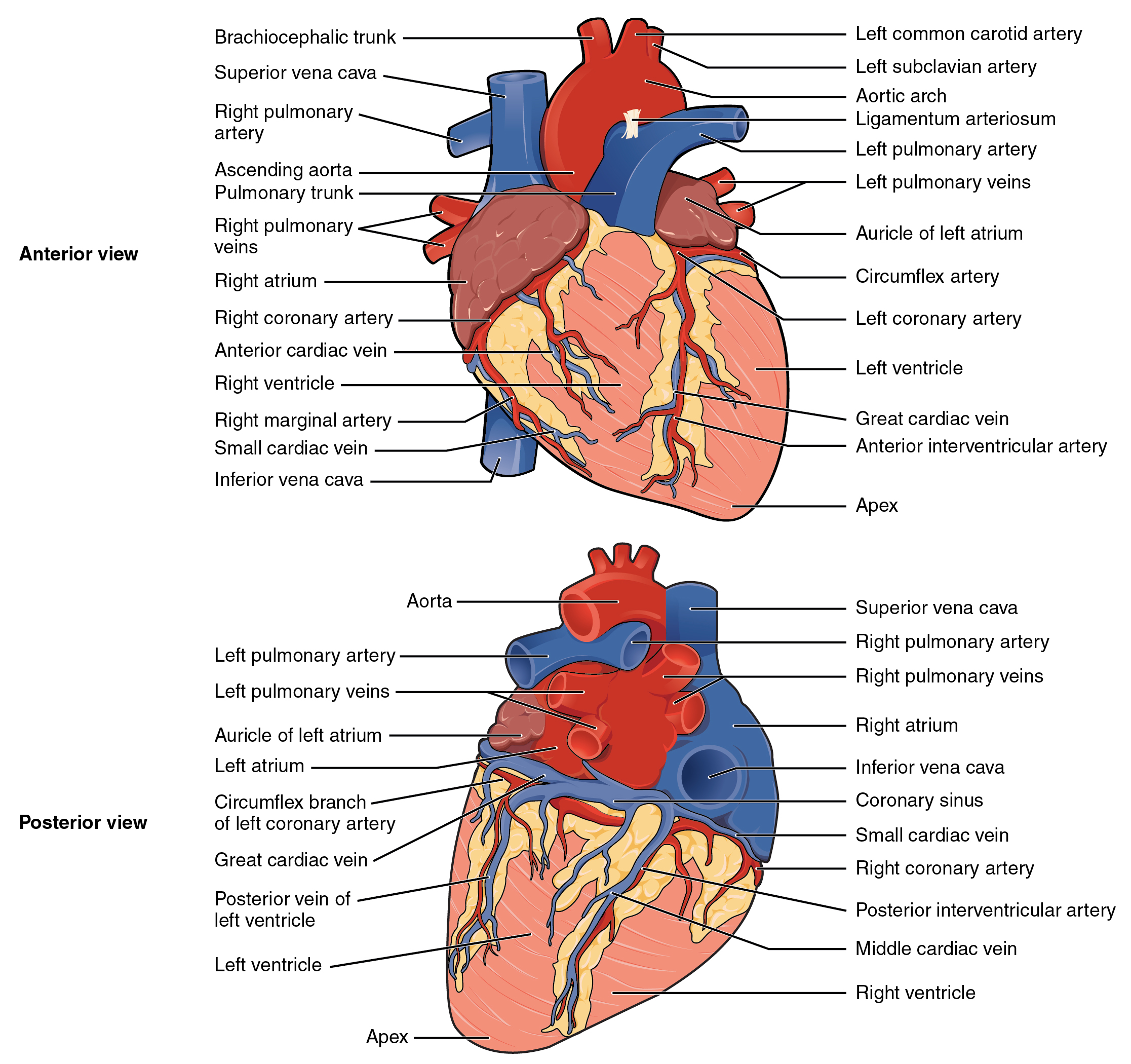
Inside the pericardium, the surface features of the heart are visible, including surface features that delineate the four chambers. There is a superficial extension of the atria near the superior surface of the heart, one on each side, called an auricle—a name that means “ear like”—because its shape resembles the external ear of a human (Figure 19.1.5). Auricles are relatively thin-walled structures that can fill with blood and empty into the atria. You may also hear them referred to as atrial appendages. Also prominent is a series of fat-filled grooves, each of which is known as a sulcus (plural = sulci). Major coronary blood vessels are located in these sulci. The deep coronary sulcus is located between the atria and ventricles and the right and left coronary arteries run in this groove. Located between the left and right ventricles are two additional sulci that are not as deep as the coronary sulcus. The anterior interventricular sulcus is visible on the anterior surface of the heart, whereas the posterior interventricular sulcus is visible on the posterior surface of the heart. These grooves contain the interventricular arteries. Figure 19.1.5 illustrates anterior and posterior views of the surface of the heart.
Layers
The wall of the heart is composed of three layers of unequal thickness. From superficial to deep, these are the epicardium, the myocardium, and the endocardium (see Figure 19.1.3). The outermost layer of the wall of the heart is also the innermost layer of the pericardium, the epicardium, or the visceral pericardium discussed earlier.
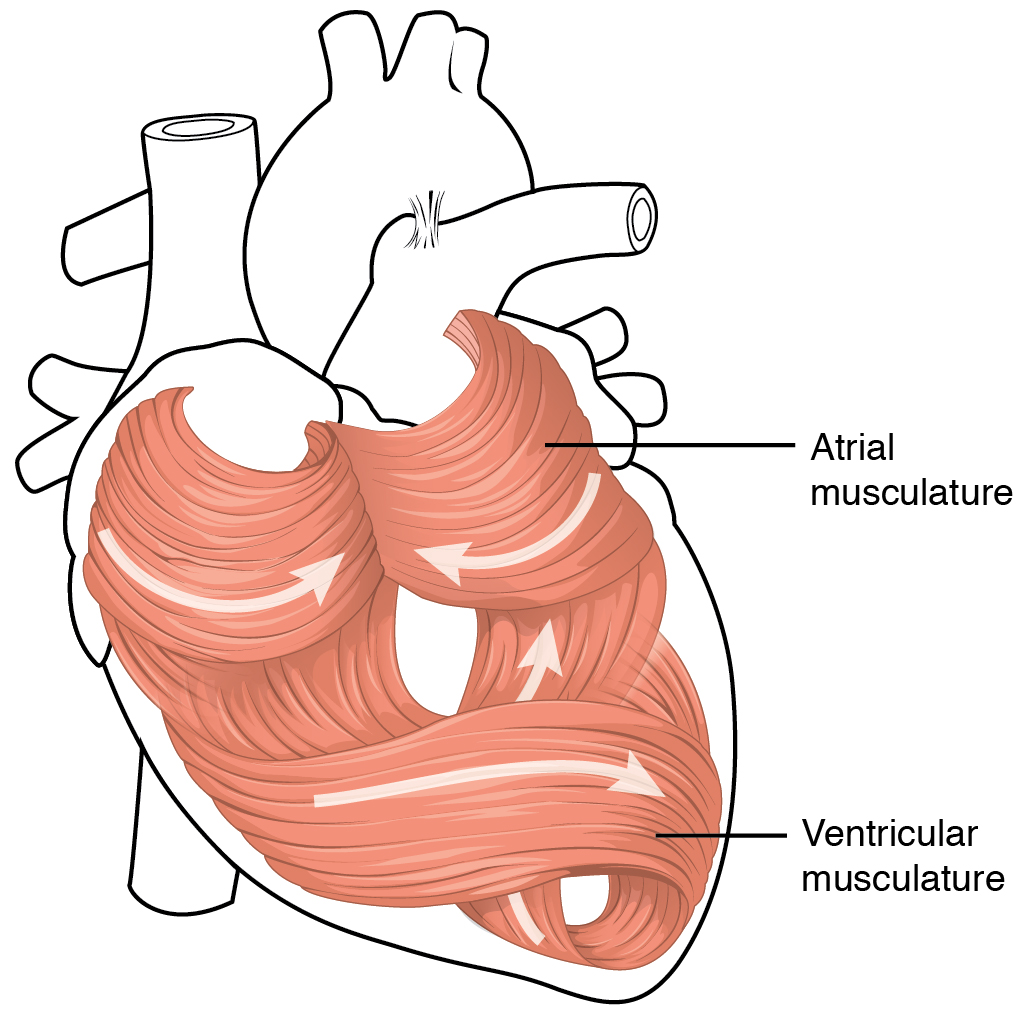
The middle and thickest layer is the myocardium, made largely of cardiac muscle cells. It is built upon a framework of collagenous fibers, plus the blood vessels that supply the myocardium and the nerve fibers that help regulate the heart. It is the contraction of the myocardium that pumps blood through the heart and into the major arteries. The muscle pattern is elegant and complex, as the muscle cells swirl and spiral around the chambers of the heart. They form a figure 8 pattern around the atria and around the bases of the great vessels. Deeper ventricular muscles also form a figure 8 around the two ventricles and proceed toward the apex. More superficial layers of ventricular muscle wrap around both ventricles(Figure 19.1.6). This complex swirling pattern allows the heart to pump blood more effectively than a simple linear pattern would (see section 19.2).
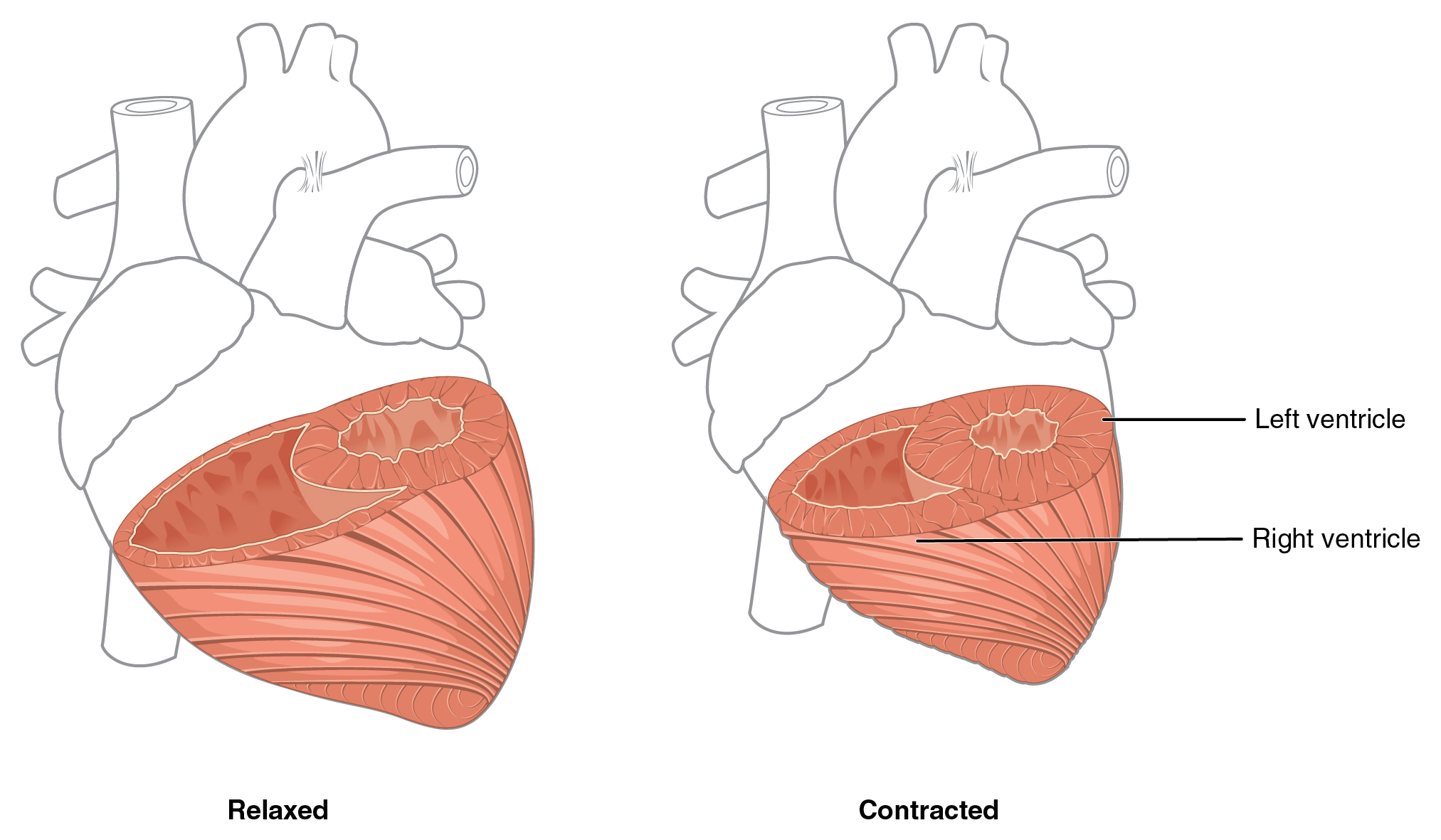
Although the ventricles on the right and left sides pump the same amount of blood per contraction, the muscle of the left ventricle is much thicker and better developed than that of the right ventricle. In order to overcome the high resistance required to pump blood into the long systemic circuit, the left ventricle must generate a great amount of pressure. The right ventricle does not need to generate as much pressure, since the pulmonary circuit is shorter and provides less resistance. Figure 19.1.7 illustrates the differences in muscular thickness needed for each of the ventricles.
The innermost layer of the heart wall, the endocardium, is joined to the myocardium with a thin layer of connective tissue. The endocardium lines the chambers where the blood circulates and covers the heart valves. It is made of simple squamous epithelium called endothelium, which is continuous with the endothelial lining of the blood vessels (see Figure 19.1.3).
Once regarded as a simple lining layer, recent evidence indicates that the endothelium of the endocardium and the coronary capillaries may play active roles in regulating the contraction of the muscle within the myocardium. The endothelium may also regulate the growth patterns of the cardiac muscle cells throughout life, and the endothelins it secretes create an environment in the surrounding tissue fluids that regulates ionic concentrations and states of contractility. Endothelins are potent vasoconstrictors and, in a normal individual, establish a homeostatic balance with other vasoconstrictors and vasodilators.
Internal Structure of the Heart
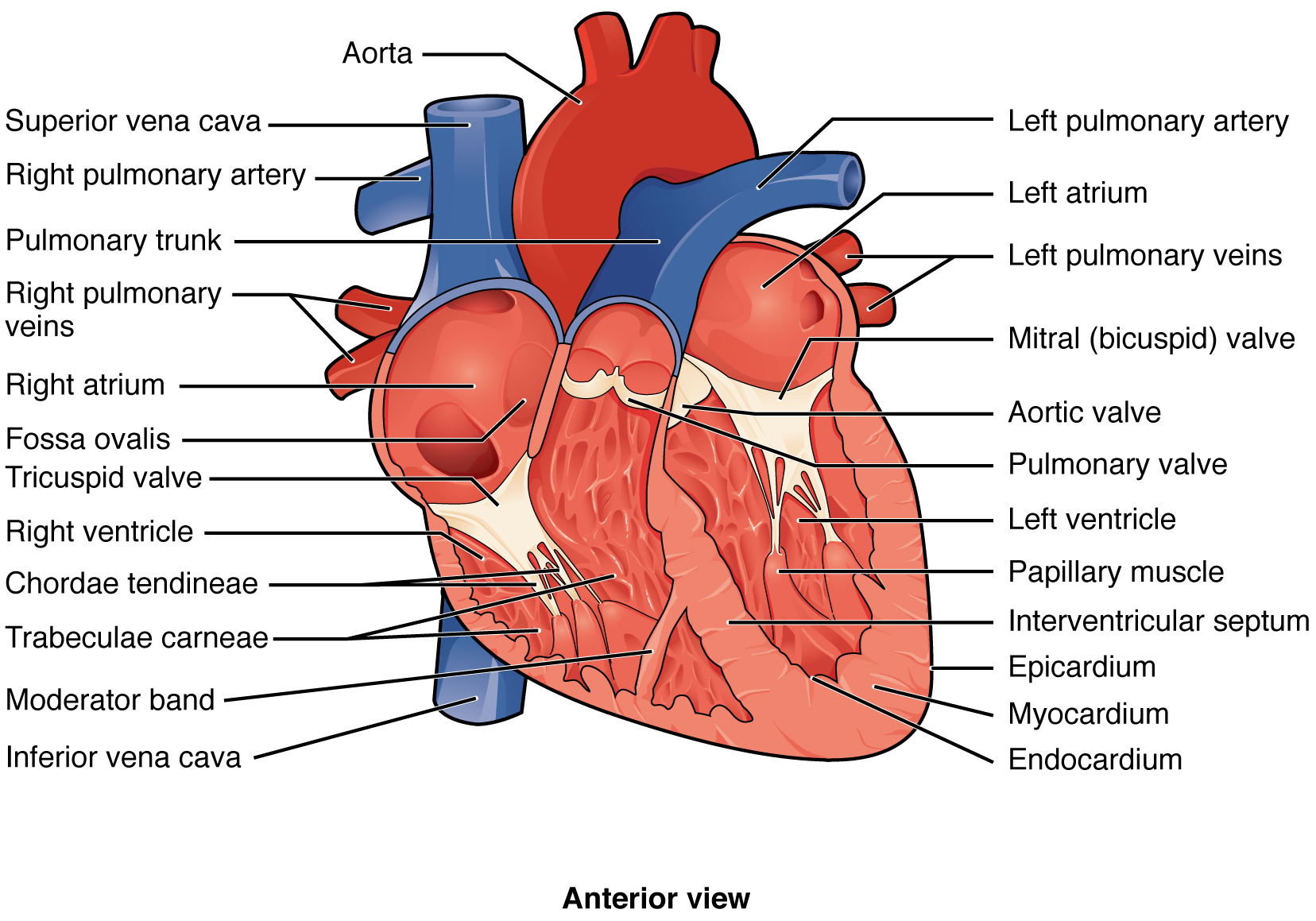
Recall that the heart’s contraction cycle follows a dual pattern of circulation—the pulmonary and systemic circuits—because the right and left pair of chambers simultaneously pump blood into the pulmonary and systemic circulations respectively. In order to develop a more precise understanding of cardiac function, it is first necessary to explore the internal anatomical structures in more detail. The majority of the internal heart structures discussed in this and subsequent sections are illustrated in Figure 19.1.8.
Septa of the Heart
The word septum is derived from the Latin for “something that encloses;” in this case, a septum (plural = septa) refers to a wall or partition that divides the heart into chambers. The septa are physical extensions of the myocardium lined with endocardium. Located between the two atria is the interatrial septum. Normally in an adult heart, the interatrial septum bears an oval-shaped depression known as the fossa ovalis, a remnant of an opening in the fetal heart known as the foramen ovale. The foramen ovale allowed blood in the fetal heart to pass directly from the right atrium to the left atrium, so some blood bypassed the pulmonary circuit. Within seconds after birth, a flap of tissue known as the septum primum, that previously acted as a valve, closes the foramen ovale and establishes the typical cardiac circulation pattern.
Between the two ventricles is a second septum known as the interventricular septum. Unlike the interatrial septum, the interventricular septum is normally intact after its formation during fetal development. It is substantially thicker than the interatrial septum, since the ventricles generate far greater pressure when they contract.
The septum between the atria and ventricles is known as the atrioventricular septum. It is marked by the presence of four openings that allow blood to move from the atria into the ventricles and from the ventricles into the pulmonary trunk and aorta. Located in each of these openings between the atria and ventricles is a valve, a specialized structure that ensures one-way flow of blood. The valves between the atria and ventricles are known generically as atrioventricular valves. The valves at the openings that lead to the pulmonary trunk and aorta are known generically as semilunar valves. The interventricular septum is visible in Figure 19.1.8. In this figure, the atrioventricular septum has been removed to better show the bicuspid (left atrioventricular) and tricuspid (right atrioventricular) valves; the interatrial septum is not visible, since its location is covered by the aorta and pulmonary trunk. Since these openings and valves structurally weaken the atrioventricular septum, the remaining tissue is heavily reinforced with dense connective tissue called the cardiac skeleton, or skeleton of the heart. It includes four rings that surround the openings between the atria and ventricles, and the openings to the pulmonary trunk and aorta, and it serves as the point of attachment for the heart valves. The cardiac skeleton also provides an important boundary in the heart electrochemical conduction system.
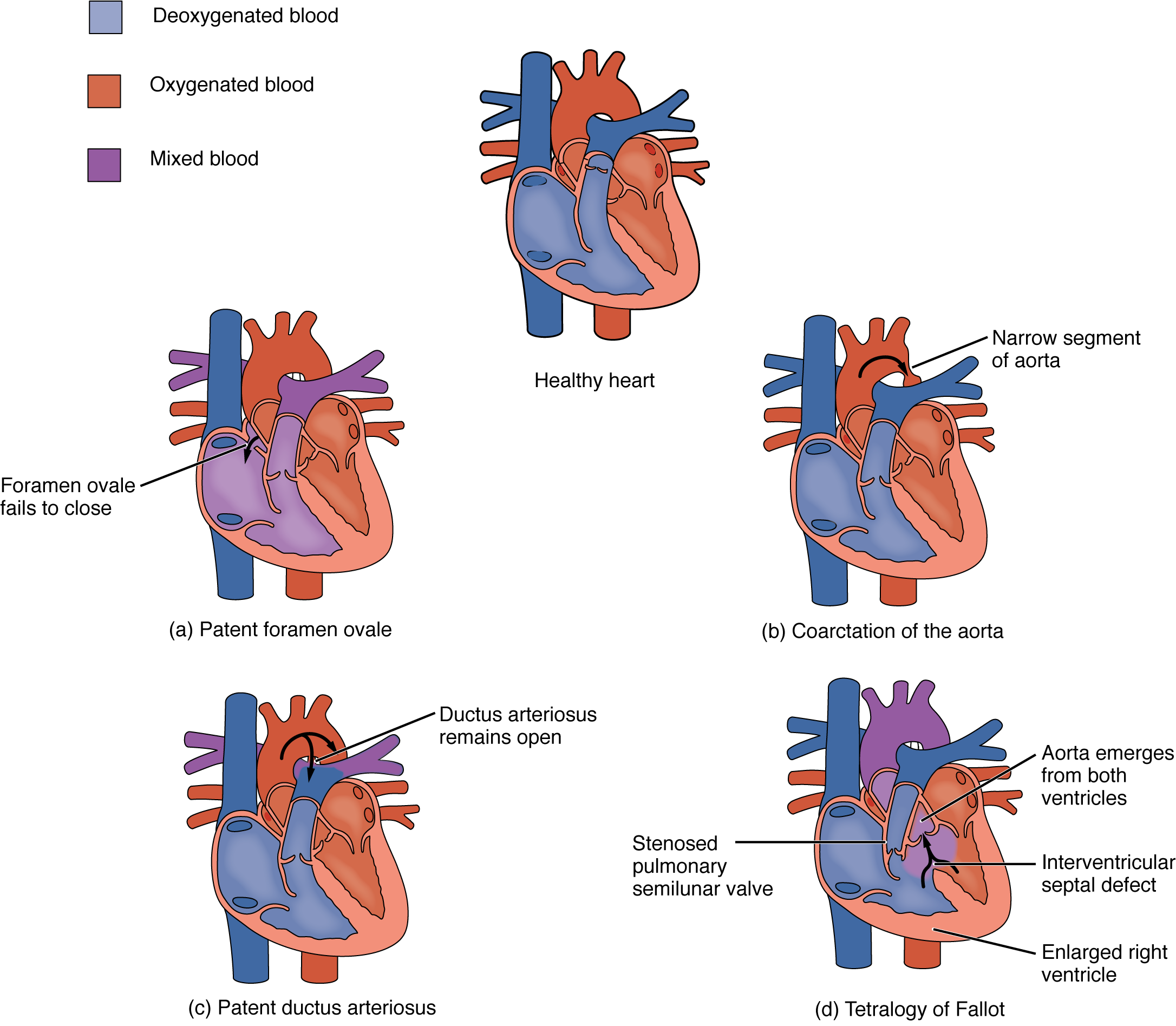
Coarctation of the aorta is a congenital abnormal narrowing of the aorta that is normally located at the insertion of the ligamentum arteriosum, the remnant of the fetal shunt called the ductus arteriosus. If severe, this condition drastically restricts blood flow through the primary systemic artery, which is life threatening. In some individuals, the condition may be fairly benign and not detected until later in life. Detectable symptoms in an infant include difficulty breathing, poor appetite, trouble feeding, or failure to thrive. In older individuals, symptoms include dizziness, fainting, shortness of breath, chest pain, fatigue, headache, and nosebleeds. Treatment involves surgery to resect (remove) the affected region or angioplasty to open the abnormally narrow passageway. Studies have shown that the earlier the surgery is performed, the better the chance of survival.
A patent ductus arteriosus is a congenital condition in which the ductus arteriosus fails to close. The condition may range from severe to benign. Failure of the ductus arteriosus to close results in blood flowing from the higher pressure aorta into the lower pressure pulmonary trunk. This additional fluid moving toward the lungs increases pulmonary pressure and makes respiration difficult. Symptoms include shortness of breath (dyspnea), tachycardia, enlarged heart, a widened pulse pressure, and poor weight gain in infants. Treatments include surgical closure (ligation), manual closure using platinum coils or specialized mesh inserted via the femoral artery or vein, or nonsteroidal anti-inflammatory drugs to block the synthesis of prostaglandin E2, which maintains the vessel in an open position. If untreated, the condition can result in congestive heart failure.
Septal defects are not uncommon in individuals and may be congenital or caused by various disease processes. Tetralogy of Fallot is a congenital condition that may also occur from exposure to unknown environmental factors; it occurs when there is an opening in the interventricular septum caused by blockage of the pulmonary trunk, normally at the pulmonary semilunar valve. This allows blood that is relatively low in oxygen from the right ventricle to flow into the left ventricle and mix with the blood that is relatively high in oxygen. Symptoms include a distinct heart murmur, low blood oxygen percent saturation, dyspnea or difficulty in breathing, polycythemia, broadening (clubbing) of the fingers and toes, and in children, difficulty in feeding or failure to grow and develop. It is the most common cause of cyanosis following birth. The term “tetralogy” is derived from the four components of the condition, although only three may be present in an individual patient: pulmonary infundibular stenosis (rigidity of the pulmonary valve), overriding aorta (the aorta is shifted above both ventricles), ventricular septal defect (opening), and right ventricular hypertrophy (enlargement of the right ventricle). Other heart defects may also accompany this condition, which is typically confirmed by echocardiography imaging. Tetralogy of Fallot occurs in approximately 400 out of one million live births. Normal treatment involves extensive surgical repair, including the use of stents to redirect blood flow and replacement of valves and patches to repair the septal defect, but the condition has a relatively high mortality. Survival rates are currently 75 percent during the first year of life; 60 percent by 4 years of age; 30 percent by 10 years; and 5 percent by 40 years.
In the case of severe septal defects, including both tetralogy of Fallot and patent foramen ovale, failure of the heart to develop properly can lead to a condition commonly known as a “blue baby.” Regardless of normal skin pigmentation, individuals with this condition have an insufficient supply of oxygenated blood, which leads to cyanosis, a blue or purple coloration of the skin, especially when active.
Septal defects are commonly first detected through auscultation, listening to the chest using a stethoscope. In this case, instead of hearing normal heart sounds attributed to the flow of blood and closing of heart valves, unusual heart sounds may be detected. This is often followed by medical imaging to confirm or rule out a diagnosis. In many cases, treatment may not be needed. Some common congenital heart defects are illustrated in Figure 19.1.9.
Right Atrium
The right atrium serves as the receiving chamber for blood returning to the heart from the systemic circulation. The two major systemic veins, the superior and inferior venae cavae, and the large coronary vein called the coronary sinus that drains the heart myocardium empty into the right atrium. The superior vena cava drains blood from regions superior to the diaphragm: the head, neck, upper limbs, and the thoracic region. It empties into the superior and posterior portions of the right atrium. The inferior vena cava drains blood from areas inferior to the diaphragm: the lower limbs and abdominopelvic region of the body. It, too, empties into the posterior portion of the atria, but inferior to the opening of the superior vena cava. Immediately superior and slightly medial to the opening of the inferior vena cava on the posterior surface of the atrium is the opening of the coronary sinus. This thin-walled vessel drains most of the coronary veins that return systemic blood from the heart (Figure 19.1.5).
While the bulk of the internal surface of the right atrium is smooth, the depression of the fossa ovalis is medial, and the anterior surface demonstrates prominent ridges of muscle called the pectinate muscles. The right auricle also has pectinate muscles. The left atrium does not have pectinate muscles except in the auricle.
The atria receive venous blood on a nearly continuous basis, preventing venous flow from stopping while the ventricles are contracting. While most ventricular filling occurs while the atria are relaxed, they do demonstrate a contractile phase and actively pump blood into the ventricles just prior to ventricular contraction. The opening between the atrium and ventricle is guarded by the tricuspid valve.
Right Ventricle
The right ventricle receives blood from the right atrium through the tricuspid valve. Each flap of the valve is attached to strong strands of connective tissue, the chordae tendineae, literally “tendinous cords,” or sometimes more poetically referred to as “heart strings.” There are several chordae tendineae associated with each of the flaps. They are composed of approximately 80 percent collagenous fibers with the remainder consisting of elastic fibers and endothelium. They connect each of the flaps to a papillary muscle that extends from the inferior ventricular surface. There are three papillary muscles in the right ventricle, called the anterior, posterior, and septal muscles, which correspond to the three sections of the valves.
When the myocardium of the ventricle contracts, pressure within the ventricular chamber rises. Blood, like any fluid, flows from higher pressure to lower pressure areas, in this case, toward the pulmonary trunk and the atrium. To prevent any potential backflow, the papillary muscles also contract, generating tension on the chordae tendineae. This prevents the flaps of the valves from being forced into the atria and regurgitation of the blood back into the atria during ventricular contraction. Figure 19.1.10 shows papillary muscles and chordae tendineae attached to the tricuspid valve.
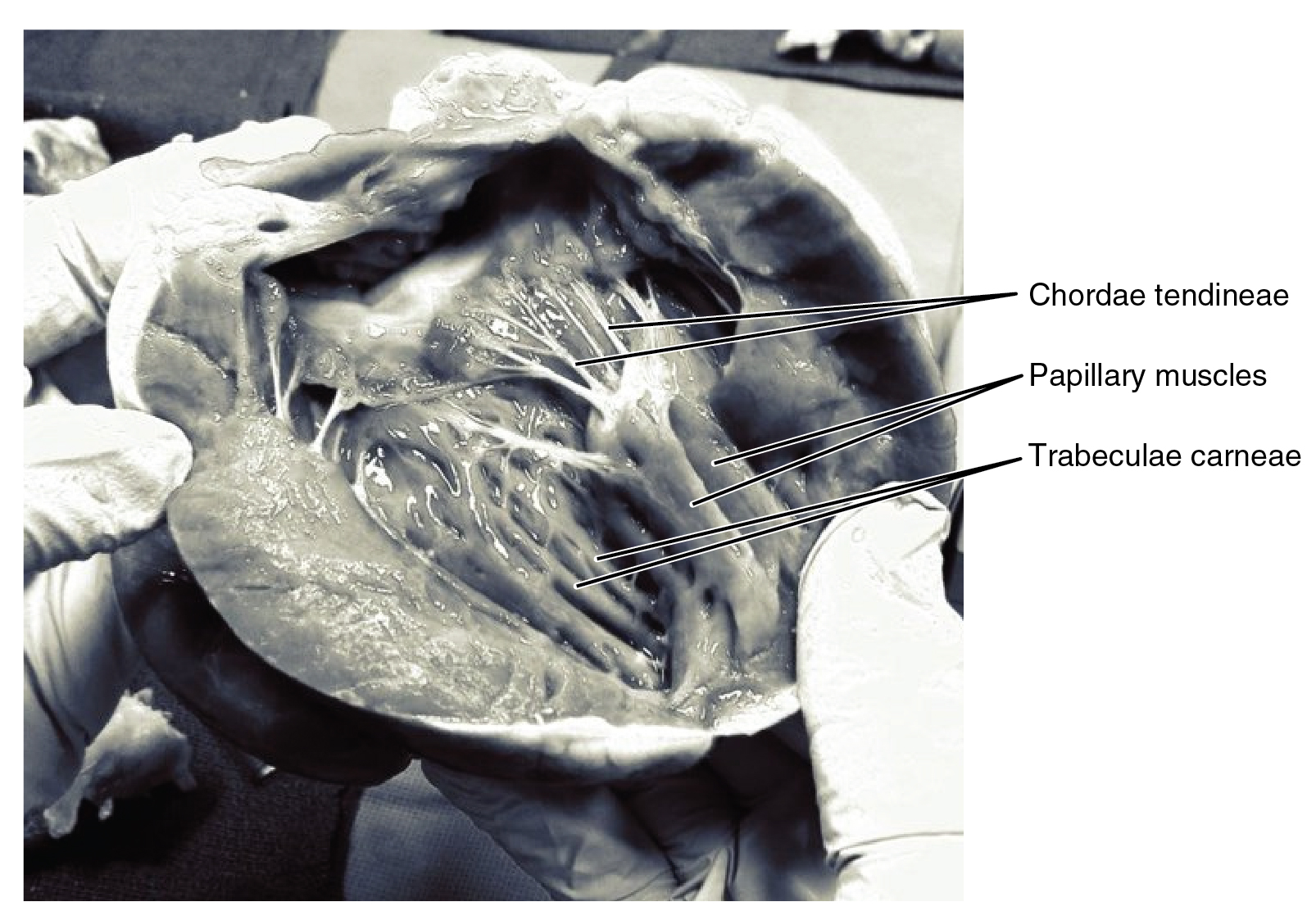
The walls of the ventricle are lined with trabeculae carneae, ridges of cardiac muscle covered by endocardium. In addition to these muscular ridges, a band of cardiac muscle, also covered by endocardium, known as the moderator band (see Figure 19.1.8) reinforces the thin walls of the right ventricle and plays a crucial role in cardiac conduction. It arises from the inferior portion of the interventricular septum and crosses the interior space of the right ventricle to connect with the inferior papillary muscle.
When the right ventricle contracts, it ejects blood into the pulmonary trunk, which branches into the left and right pulmonary arteries that carry it to each lung. The superior surface of the right ventricle begins to taper as it approaches the pulmonary trunk. At the base of the pulmonary trunk is the pulmonary semilunar valve that prevents backflow from the pulmonary trunk.
Left Atrium
After exchange of gases in the pulmonary capillaries, blood returns to the left atrium high in oxygen via one of the four pulmonary veins. While the left atrium does not contain pectinate muscles, it does have an auricle that includes these pectinate ridges. Blood flows nearly continuously from the pulmonary veins back into the atrium, which acts as the receiving chamber, and from here through an opening into the left ventricle when the ventricle is relaxed. The opening between the left atrium and ventricle is guarded by the mitral or bicuspid valve.
Left Ventricle
Recall that, although both sides of the heart will pump the same amount of blood, the muscular layer is much thicker in the left ventricle compared to the right (see Figure 19.1.7). Like the right ventricle, the left also has trabeculae carneae, but there is no moderator band. The mitral valve is connected to papillary muscles via chordae tendineae. There are two papillary muscles on the left—the anterior and posterior—as opposed to three on the right.
The left ventricle is the major pumping chamber for the systemic circuit; it ejects blood into the aorta through the aortic semilunar valve.
Heart Valve Structure and Function
A transverse section through the heart slightly above the level of the atrioventricular septum reveals all four heart valves along the same plane (Figure 19.1.11). The valves ensure unidirectional blood flow through the heart. Between the right atrium and the right ventricle is the right atrioventricular valve, or tricuspid valve. It typically consists of three flaps, or leaflets, made of endocardium reinforced with additional connective tissue. The flaps are connected by chordae tendineae to the papillary muscles, which control the opening and closing of the valves.
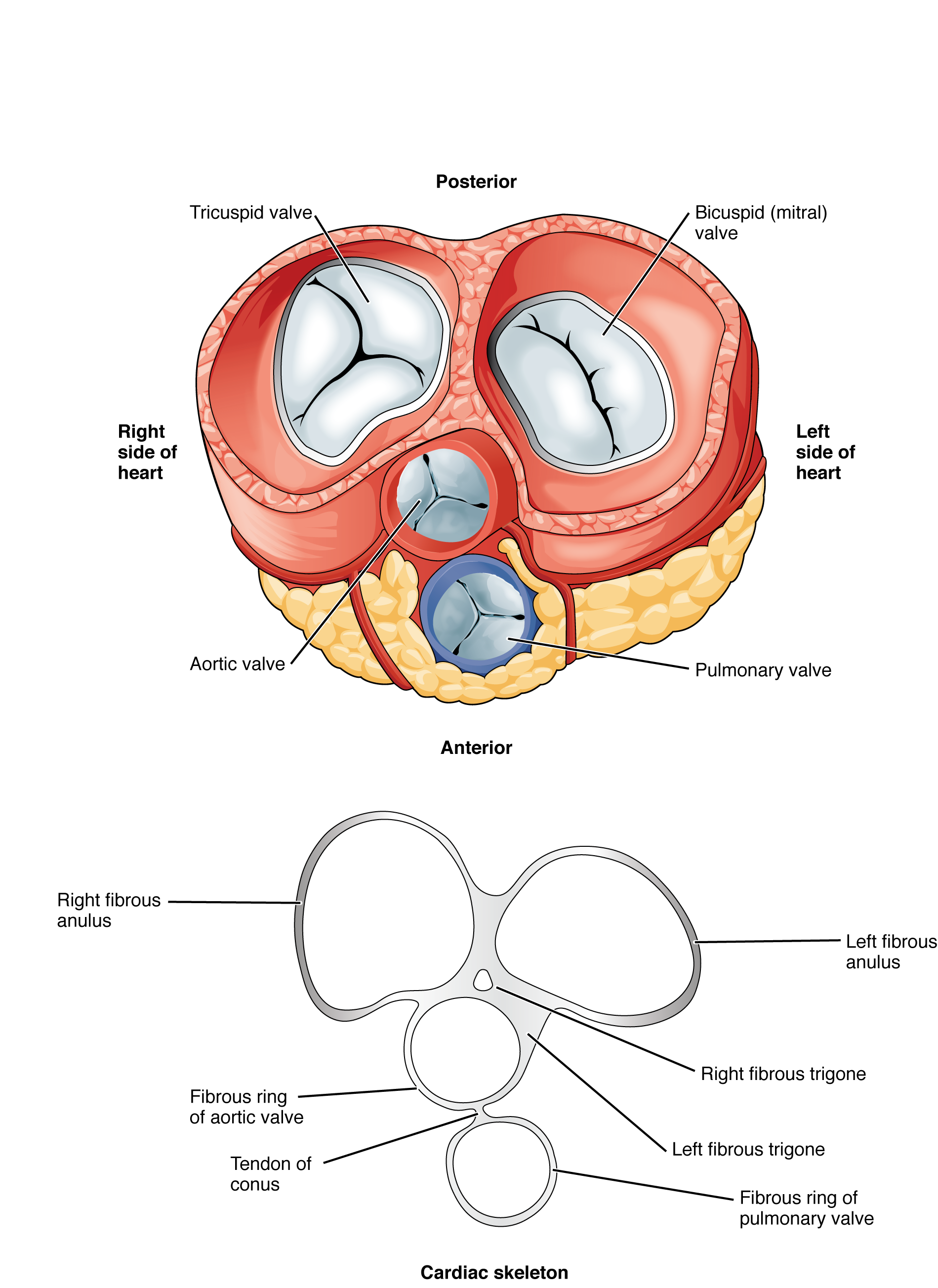
Emerging from the right ventricle at the base of the pulmonary trunk is the pulmonary semilunar valve, or the pulmonary valve; it is also known as the pulmonic valve or the right semilunar valve. The pulmonary valve is comprised of three small flaps of endothelium reinforced with connective tissue. When the ventricle relaxes, the pressure differential causes blood to flow back into the ventricle from the pulmonary trunk. This flow of blood fills the pocket-like flaps of the pulmonary valve, causing the valve to close and producing an audible sound. Unlike the atrioventricular valves, there are no papillary muscles or chordae tendineae associated with the pulmonary valve.
Located at the opening between the left atrium and left ventricle is the mitral valve, also called the bicuspid valve or the left atrioventricular valve. Structurally, this valve consists of two cusps, known as the anterior medial cusp and the posterior medial cusp, compared to the three cusps of the tricuspid valve. In a clinical setting, the valve is referred to as the mitral valve, rather than the bicuspid valve. The two cusps of the mitral valve are attached by chordae tendineae to two papillary muscles that project from the wall of the ventricle.
At the base of the aorta is the aortic semilunar valve, or the aortic valve, which prevents backflow from the aorta. It normally is composed of three flaps. When the ventricle relaxes and blood attempts to flow back into the ventricle from the aorta, blood will fill the cusps of the valve, causing it to close and producing an audible sound.
In Figure 19.1.12a, the two atrioventricular valves are open and the two semilunar valves are closed. This occurs when both atria and ventricles are relaxed and when the atria contract to pump blood into the ventricles. Figure 19.1.12b shows a frontal view. Although only the left side of the heart is illustrated, the process is virtually identical on the right.
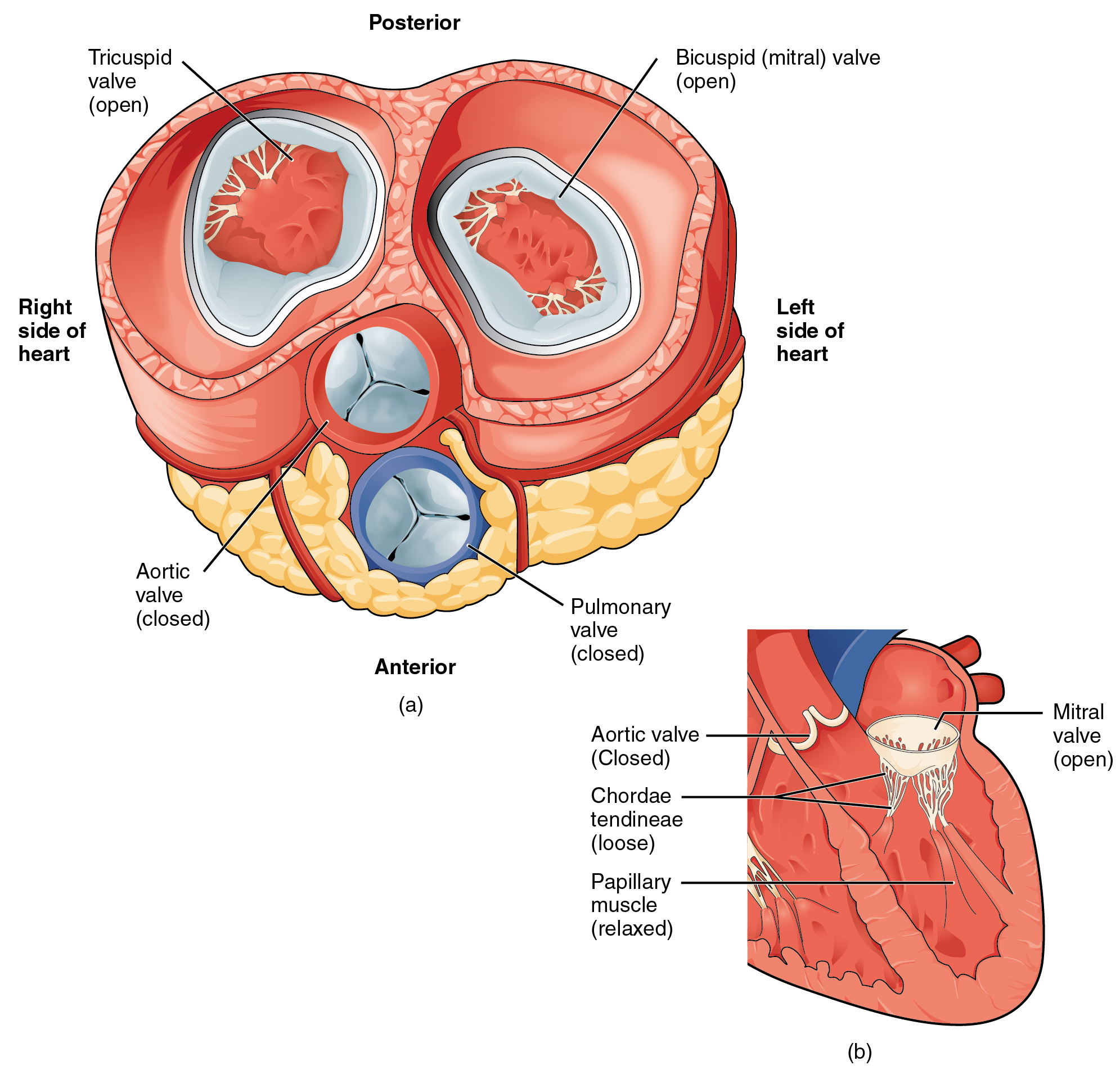
Figure 19.1.13a shows the atrioventricular valves closed while the two semilunar valves are open. This occurs when the ventricles contract to eject blood into the pulmonary trunk and aorta. Closure of the two atrioventricular valves prevents blood from being forced back into the atria. This stage can be seen from a frontal view in Figure 19.1.13b.
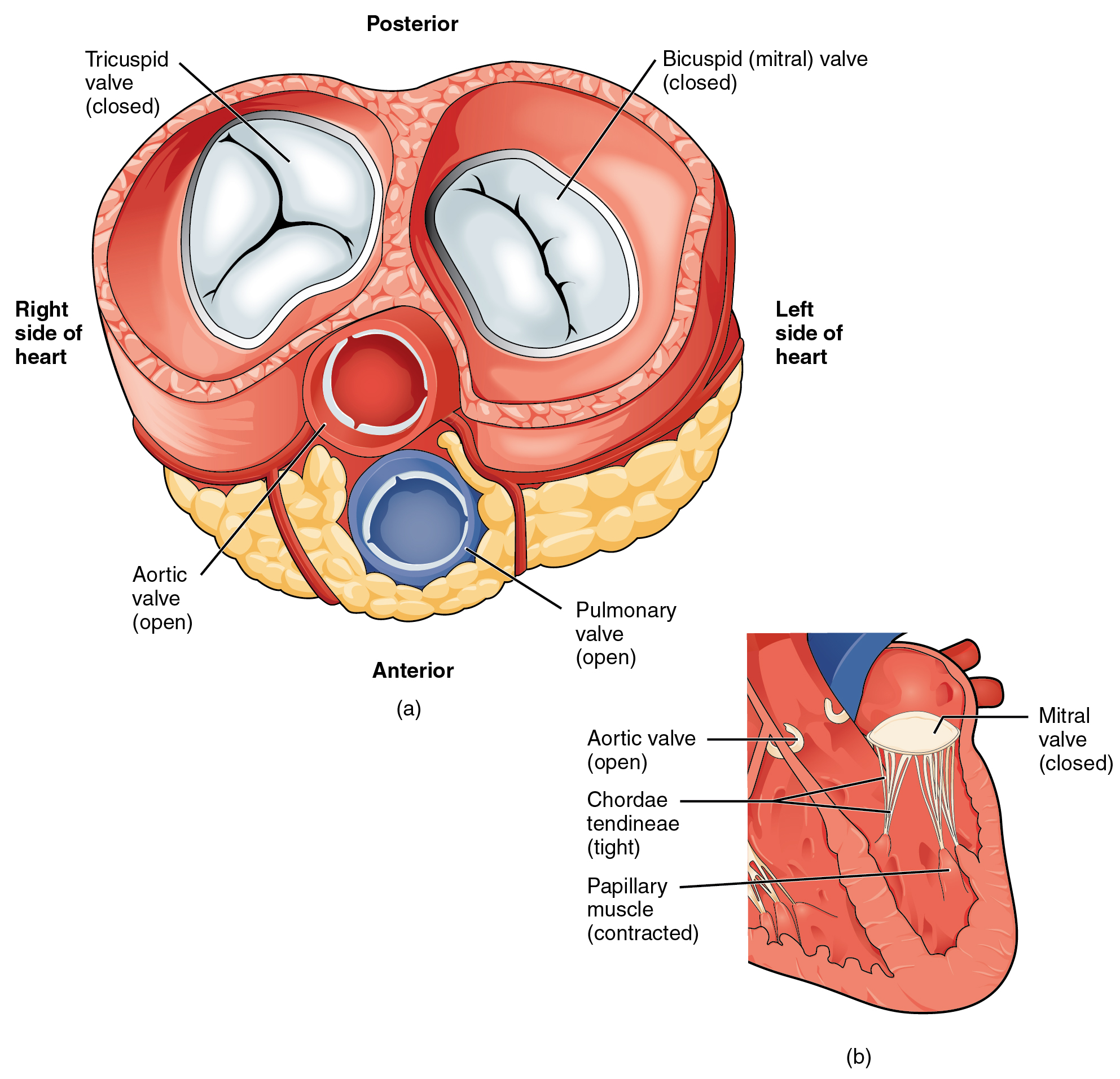
When the ventricles begin to contract, pressure within the ventricles rises and blood flows toward the area of lowest pressure, which is initially in the atria. This backflow causes the cusps of the tricuspid and mitral (bicuspid) valves to close. These valves are tied down to the papillary muscles by chordae tendineae. During the relaxation phase of the cardiac cycle, the papillary muscles are also relaxed and the tension on the chordae tendineae is slight (see Figure 19.1.12b). However, as the myocardium of the ventricle contracts, so do the papillary muscles. This creates tension on the chordae tendineae (see Figure 19.1.13b), helping to hold the cusps of the atrioventricular valves in place and preventing them from being blown back into the atria.
The aortic and pulmonary semilunar valves lack the chordae tendineae and papillary muscles associated with the atrioventricular valves. Instead, they consist of pocket-like folds of endocardium reinforced with additional connective tissue. When the ventricles relax and the change in pressure forces the blood toward the ventricles, the blood presses against these cusps and seals the openings.
External Website

Visit this site to observe an echocardiogram of actual heart valves opening and closing. Although much of the heart has been “removed” from this gif loop so the chordae tendineae are not visible, why is their presence more critical for the atrioventricular valves (tricuspid and mitral) than the semilunar (aortic and pulmonary) valves?
When heart valves do not close properly, they are often described as incompetent or insufficient and this leads to the backflow or regurgitation of blood. The resulting valvular heart disease can range from benign to lethal. Some of these conditions are congenital, that is, the individual was born with the defect, whereas others may be attributed to disease processes or trauma. Some malfunctions are treated with medications, others require surgery, and still others may be mild enough that the condition is merely monitored since treatment might trigger more serious consequences.
Valvular disorders are often caused by carditis, or inflammation of the heart. One common trigger for this inflammation is rheumatic fever, or scarlet fever, an autoimmune response due to the production of antibodies to the bacterium, Streptococcus pyogenes (cause of Strep throat).
While any of the heart valves may be involved in valve disorders, mitral regurgitation is the most common, detected in approximately 2 percent of the population, and the pulmonary semilunar valve is the least frequently involved. When a valve malfunctions, the flow of blood to a region will often be disrupted. The resulting inadequate flow of blood to this region will be described in general terms as an insufficiency. The specific type of insufficiency is named for the valve involved: aortic insufficiency, mitral insufficiency, tricuspid insufficiency, or pulmonary insufficiency.
If one of the cusps of the valve is forced backward by the force of the blood, the condition is referred to as a prolapsed valve. Prolapse may occur if the chordae tendineae are damaged or broken, causing the closure mechanism to fail. The failure of the valve to close properly disrupts the normal one-way flow of blood and results in regurgitation, when the blood flows backward from its normal path. Using a stethoscope, the disruption to the normal flow of blood produces a heart murmur.
Stenosis is a condition in which the heart valves become rigid and may calcify over time. The loss of flexibility of the valve interferes with normal function and may cause the heart to work harder to propel blood through the valve, which eventually weakens the heart. Aortic stenosis affects approximately 2 percent of the population over 65 years of age, and the percentage increases to approximately 4 percent in individuals over 85 years. Occasionally, one or more of the chordae tendineae will tear or the papillary muscle itself may die as a component of a myocardial infarction (heart attack). In this case, the patient’s condition will deteriorate dramatically and rapidly, and immediate surgical intervention may be required.
Auscultation, or listening to a patient’s heart sounds, is one of the most useful diagnostic tools, since it is proven, safe, and inexpensive. The term auscultation is derived from the Latin for “to listen,” and the technique has been used for diagnostic purposes as far back as the ancient Egyptians. Valve and septal disorders will trigger abnormal heart sounds. If a valvular disorder is detected or suspected, a test called an echocardiogram, or simply an “echo,” may be ordered. Echocardiograms are sonograms of the heart and can help in the diagnosis of valve disorders as well as a wide variety of heart pathologies.
External Website

Visit this site for a free download, including excellent animations and audio of heart sounds.
Career Connection – Cardiologist
Cardiologists are medical doctors that specialize in the diagnosis and treatment of diseases of the heart. After completing 4 years of medical school, cardiologists complete a three-year residency in internal medicine followed by an additional three or more years in cardiology. Following this 10-year period of medical training and clinical experience, they qualify for a rigorous two-day examination administered by the Board of Internal Medicine that tests their academic training and clinical abilities, including diagnostics and treatment. After successful completion of this examination, a physician becomes a board-certified cardiologist. Some board-certified cardiologists may be invited to become a Fellow of the American College of Cardiology (FACC). This professional recognition is awarded to outstanding physicians based upon merit, including outstanding credentials, achievements, and community contributions to cardiovascular medicine.
Career Connection – Cardiovascular Technologist/Technician
Cardiovascular technologists/technicians are trained professionals who perform a variety of imaging techniques, such as sonograms or echocardiograms, used by physicians to diagnose and treat diseases of the heart. Nearly all of these positions require an associate degree, and these technicians earn a median salary of $49,410 as of May 2010, according to the U.S. Bureau of Labor Statistics. Growth within the field is fast, projected at 29 percent from 2010 to 2020.
There is a considerable overlap and complementary skills between cardiac technicians and vascular technicians, and so the term cardiovascular technician is often used. Special certifications within the field require documenting appropriate experience and completing additional and often expensive certification examinations. These subspecialties include Certified Rhythm Analysis Technician (CRAT), Certified Cardiographic Technician (CCT), Registered Congenital Cardiac Sonographer (RCCS), Registered Cardiac Electrophysiology Specialist (RCES), Registered Cardiovascular Invasive Specialist (RCIS), Registered Cardiac Sonographer (RCS), Registered Vascular Specialist (RVS), and Registered Phlebology Sonographer (RPhS).
Coronary Circulation
You will recall that the heart is a remarkable pump composed largely of cardiac muscle cells that are incredibly active throughout life. Like all other cells, a cardiomyocyte requires a reliable supply of oxygen and nutrients, and a way to remove wastes, so it needs a dedicated, complex, and extensive coronary circulation to supply the thick myocardium. And because of the critical and nearly ceaseless activity of the heart throughout life, this need for a blood supply is even greater than for a typical cell. However, coronary circulation is not continuous; rather, it cycles, reaching a peak when the heart muscle is relaxed and nearly ceasing while it is contracting.
Coronary Arteries
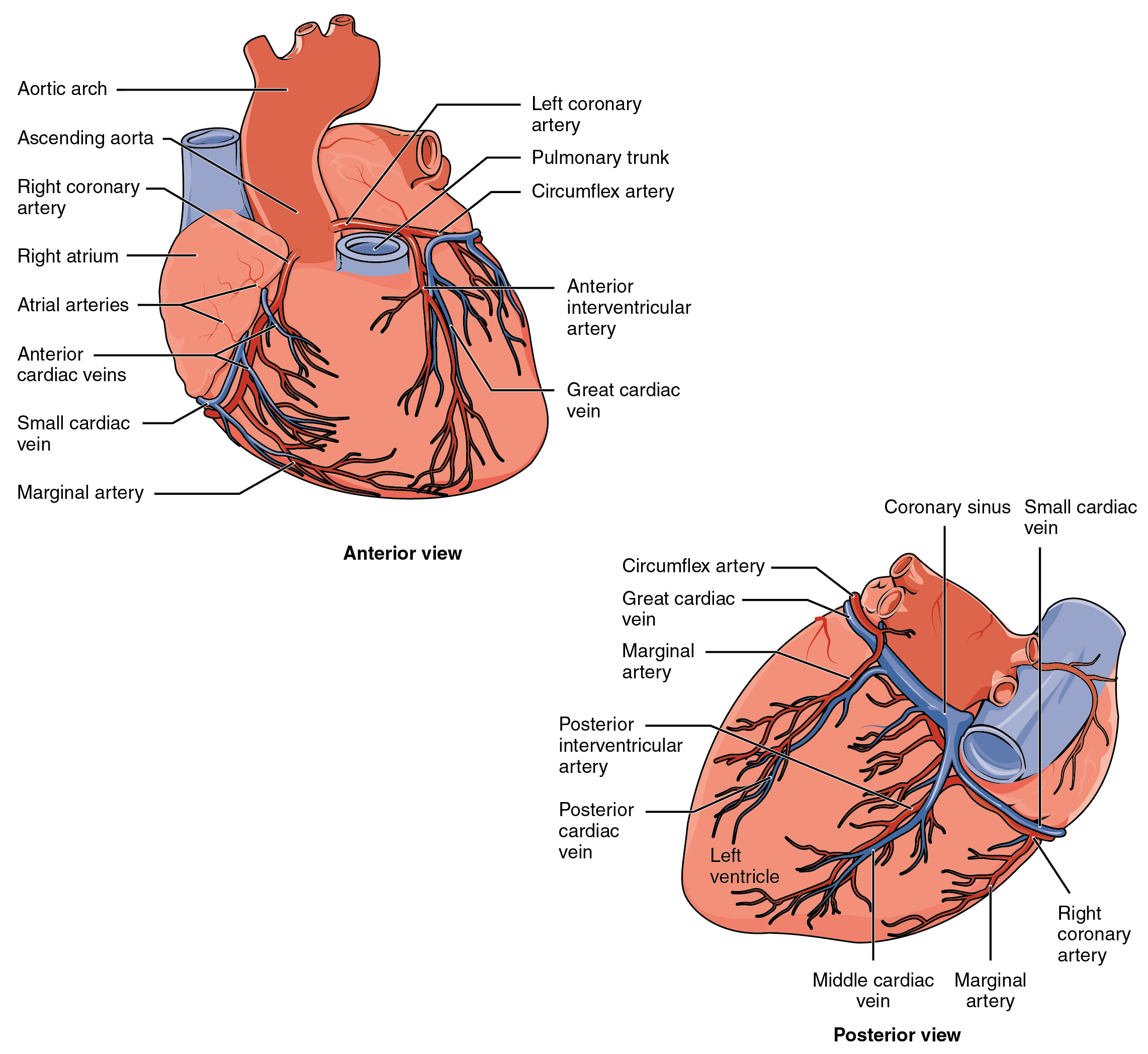
Coronary arteries supply blood to the myocardium and other components of the heart. The first portion of the aorta after it arises from the left ventricle gives rise to the coronary arteries. There are three dilations in the wall of the aorta just superior to the aortic semilunar valve. Two of these, the left posterior aortic sinus and anterior aortic sinus, give rise to the left and right coronary arteries, respectively. The third sinus, the right posterior aortic sinus, typically does not give rise to a vessel. Coronary vessel branches that remain on the surface of the artery and follow the sulci are called epicardial coronary arteries.
The left coronary artery distributes blood to the left side of the heart, the left atrium and ventricle, and the interventricular septum. The circumflex artery arises from the left coronary artery and follows the coronary sulcus to the left. Eventually, it will fuse with the small branches of the right coronary artery. The larger anterior interventricular artery, also known as the left anterior descending artery (LAD), is the second major branch arising from the left coronary artery. It follows the anterior interventricular sulcus around the pulmonary trunk. Along the way it gives rise to numerous smaller branches that interconnect with the branches of the posterior interventricular artery, forming anastomoses. An anastomosis is an area where vessels unite to form interconnections that normally allow blood to circulate to a region even if there may be partial blockage in another branch. The anastomoses in the heart are very small. Therefore, this ability is somewhat restricted in the heart so a coronary artery blockage often results in death of the cells (myocardial infarction) supplied by the particular vessel.
The right coronary artery proceeds along the coronary sulcus and distributes blood to the right atrium, portions of both ventricles, and the heart conduction system. Normally, one or more marginal arteries arise from the right coronary artery inferior to the right atrium. The marginal arteries supply blood to the superficial portions of the right ventricle. On the posterior surface of the heart, the right coronary artery gives rise to the posterior interventricular artery, also known as the posterior descending artery. It runs along the posterior portion of the interventricular sulcus toward the apex of the heart, giving rise to branches that supply the interventricular septum and portions of both ventricles. Figure 19.1.14 presents views of the coronary circulation from both the anterior and posterior views.
In the case of acute MI, there is often sudden pain beneath the sternum (retrosternal pain) called angina pectoris, often radiating down the left arm in males but not in female patients. Until this anomaly between the sexes was discovered, many female patients suffering MIs were misdiagnosed and sent home. In addition, patients typically present with difficulty breathing and shortness of breath (dyspnea), irregular heartbeat (palpations), nausea and vomiting, sweating (diaphoresis), anxiety, and fainting (syncope), although not all of these symptoms may be present. Many of the symptoms are shared with other medical conditions, including anxiety attacks and simple indigestion, so differential diagnosis is critical. It is estimated that between 22 and 64 percent of MIs present without any symptoms.
An MI can be confirmed by examining the patient’s ECG, which frequently reveals alterations in the ST and Q components. Some classification schemes of MI are referred to as ST-elevated MI (STEMI) and non-elevated MI (non-STEMI). In addition, echocardiography or cardiac magnetic resonance imaging may be employed. Common blood tests indicating an MI include elevated levels of creatine kinase MB (an enzyme that catalyzes the conversion of creatine to phosphocreatine, consuming ATP) and cardiac troponin (the regulatory protein for muscle contraction), both of which are released by damaged cardiac muscle cells.
Immediate treatments for MI are essential and include administering supplemental oxygen, aspirin that prevents the clot from enlarging, and nitroglycerine administered sublingually (under the tongue) to facilitate its absorption. Despite its unquestioned success in treatments and use since the 1880s, the mechanism of nitroglycerine is still incompletely understood but is believed to involve the release of nitric oxide, a known vasodilator, and endothelium-derived releasing factor, which also relaxes the smooth muscle in the tunica media of coronary vessels. Longer-term treatments include injections of thrombolytic agents such as streptokinase that dissolve the clot, the anticoagulant heparin, balloon angioplasty and stents to open blocked vessels, and bypass surgery to allow blood to pass around the site of blockage. If the damage is extensive, coronary replacement with a donor heart or coronary assist device, a sophisticated mechanical device that supplements the pumping activity of the heart, may be employed. Despite the attention, development of artificial hearts to augment the severely limited supply of heart donors has proven less than satisfactory but will likely improve in the future.
MIs may trigger cardiac arrest, but the two are not synonymous. Important risk factors for MI include cardiovascular disease, age, smoking, high blood levels of the low-density lipoprotein (LDL, often referred to as “bad” cholesterol), low levels of high-density lipoprotein (HDL, or “good” cholesterol), hypertension, diabetes mellitus, obesity, lack of physical exercise, chronic kidney disease, excessive alcohol consumption, and use of illegal drugs.
Coronary Veins
Coronary veins drain the heart and generally parallel the large surface arteries (see Figure 19.1.14). The great cardiac vein can be seen initially on the surface of the heart following the interventricular sulcus, but it eventually flows along the coronary sulcus into the coronary sinus on the posterior surface. The great cardiac vein initially parallels the anterior interventricular artery and drains the areas supplied by this vessel. It receives several major branches, including the posterior cardiac vein, the middle cardiac vein, and the small cardiac vein. The posterior cardiac vein parallels and drains the areas supplied by the marginal artery branch of the circumflex artery. The middle cardiac vein parallels and drains the areas supplied by the posterior interventricular artery. The small cardiac vein parallels the right coronary artery and drains the blood from the posterior surfaces of the right atrium and ventricle. The coronary sinus is a large, thin-walled vein on the posterior surface of the heart lying within the atrioventricular sulcus and emptying directly into the right atrium. The anterior cardiac veins parallel the small cardiac arteries and drain the anterior surface of the right ventricle. Unlike these other cardiac veins, it bypasses the coronary sinus and drains directly into the right atrium.
Coronary artery disease is the leading cause of death worldwide. It occurs when the buildup of plaque—a fatty material including cholesterol, connective tissue, macrophages, and some smooth muscle cells—within the walls of the arteries obstructs the flow of blood and decreases the flexibility or compliance of the vessels. This condition is called atherosclerosis, a hardening of the arteries that involves the accumulation of plaque. As the coronary blood vessels become occluded, the flow of blood to the tissues will be restricted, a condition called ischemia that causes the cells to receive insufficient amounts of oxygen, called hypoxia. Figure 19.1.15 shows the blockage of coronary arteries highlighted by the injection of dye. Some individuals with coronary artery disease report pain radiating from the chest called angina pectoris, but others remain asymptomatic. If untreated, coronary artery disease can lead to MI or a heart attack.
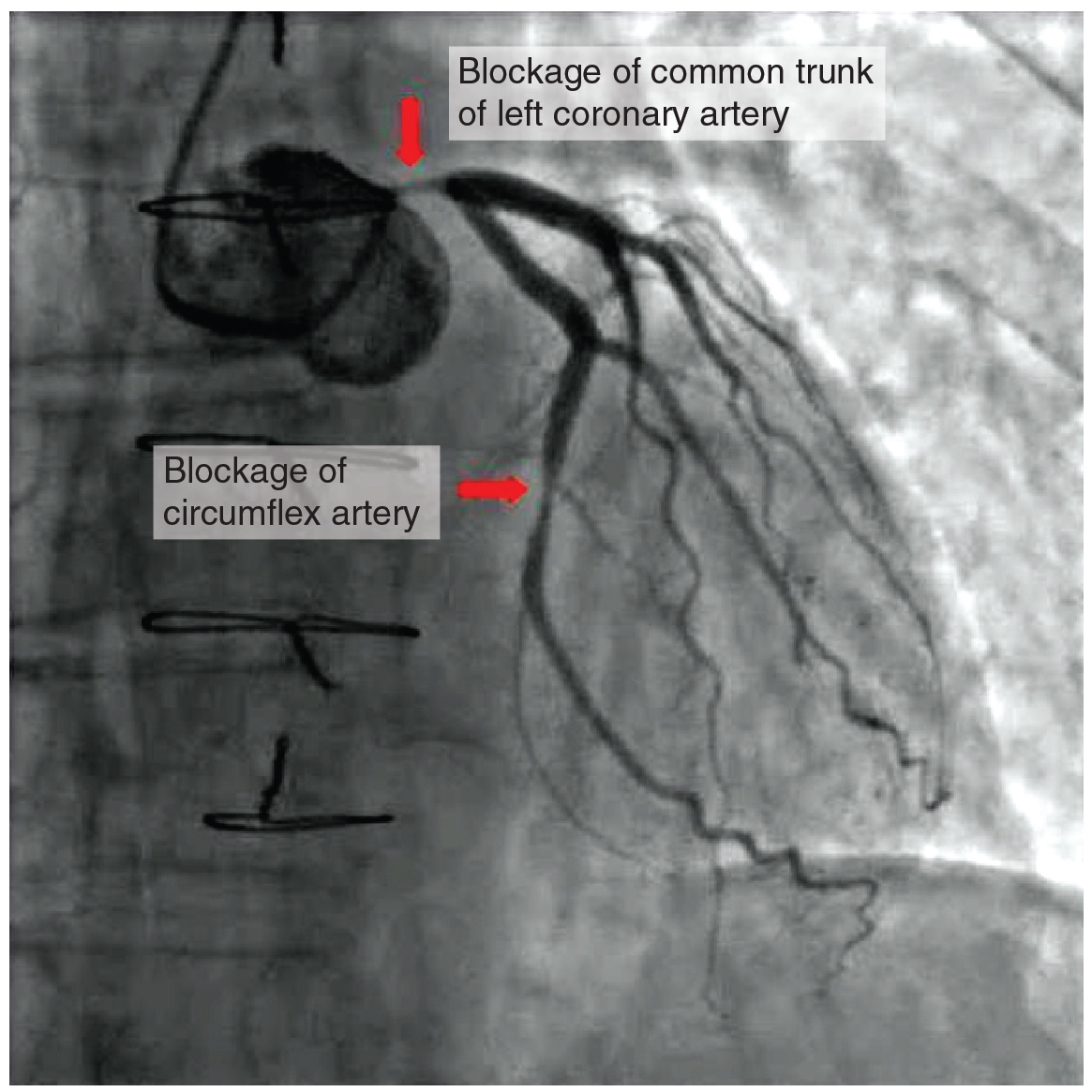
The disease progresses slowly and often begins in children and can be seen as fatty “streaks” in the vessels. It then gradually progresses throughout life. Well-documented risk factors include smoking, family history, hypertension, obesity, diabetes, high alcohol consumption, lack of exercise, stress, and hyperlipidemia or high circulating levels of lipids in the blood. Treatments may include medication, changes to diet and exercise, angioplasty with a balloon catheter, insertion of a stent, or coronary bypass procedure.
Angioplasty is a procedure in which the occlusion is mechanically widened with a balloon. A specialized catheter with an expandable tip is inserted into a superficial vessel, normally in the leg, and then directed to the site of the occlusion. At this point, the balloon is inflated to compress the plaque material and to open the vessel to increase blood flow. Then, the balloon is deflated and retracted. A stent consisting of a specialized mesh is typically inserted at the site of occlusion to reinforce the weakened and damaged walls. Stent insertions have been routine in cardiology for more than 40 years.
Coronary bypass surgery may also be performed. This surgical procedure grafts a replacement vessel obtained from another, less vital portion of the body to bypass the occluded area. This procedure is clearly effective in treating patients experiencing a MI, but overall does not increase longevity. Nor does it seem advisable in patients with stable although diminished cardiac capacity since frequently loss of mental acuity occurs following the procedure. Long-term changes to behavior, emphasizing diet and exercise plus a medicine regime tailored to lower blood pressure, lower cholesterol and lipids, and reduce clotting are equally as effective.
Chapter Review
The heart resides within the pericardial sac and is located in the mediastinal space within the thoracic cavity. The pericardial sac consists of two fused layers: an outer fibrous capsule and an inner parietal pericardium lined with a serous membrane. Between the pericardial sac and the heart is the pericardial cavity, which is filled with lubricating serous fluid. The walls of the heart are composed of an outer epicardium, a thick myocardium, and an inner lining layer of endocardium. The human heart consists of a pair of atria, which receive blood and pump it into a pair of ventricles, which pump blood into the vessels. The right atrium receives systemic blood relatively low in oxygen and pumps it into the right ventricle, which pumps it into the pulmonary circuit. Exchange of oxygen and carbon dioxide occurs in the lungs, and blood high in oxygen returns to the left atrium, which pumps blood into the left ventricle, which in turn pumps blood into the aorta and the remainder of the systemic circuit. The septa are the partitions that separate the chambers of the heart. They include the interatrial septum, the interventricular septum, and the atrioventricular septum. Two of these openings are guarded by the atrioventricular valves, the right tricuspid valve and the left mitral valve, which prevent the backflow of blood. Each is attached to chordae tendineae that extend to the papillary muscles, which are extensions of the myocardium, to prevent the valves from being blown back into the atria. The pulmonary valve is located at the base of the pulmonary trunk, and the left semilunar valve is located at the base of the aorta. The right and left coronary arteries are the first to branch off the aorta and arise from two of the three sinuses located near the base of the aorta and are generally located in the sulci. Cardiac veins parallel the small cardiac arteries and generally drain into the coronary sinus.
Interactive Link Questions
Visit this site to observe an echocardiogram of actual heart valves opening and closing. Although much of the heart has been “removed” from this gif loop so the chordae tendineae are not visible, why is their presence more critical for the atrioventricular valves (tricuspid and mitral) than the semilunar (aortic and pulmonary) valves?
The pressure gradient between the atria and the ventricles is much greater than that between the ventricles and the pulmonary trunk and aorta. Without the presence of the chordae tendineae and papillary muscles, the valves would be blown back (prolapsed) into the atria and blood would regurgitate.
Review Questions
Critical Thinking Questions
1. Describe how the valves keep the blood moving in one direction.
2. Why is the pressure in the pulmonary circulation lower than in the systemic circulation?
Glossary
- anastomosis
- (plural = anastomoses) area where vessels unite to allow blood to circulate even if there may be partial blockage in another branch
- anterior cardiac veins
- vessels that parallel the small cardiac arteries and drain the anterior surface of the right ventricle; bypass the coronary sinus and drain directly into the right atrium
- anterior interventricular artery
- (also, left anterior descending artery or LAD) major branch of the left coronary artery that follows the anterior interventricular sulcus
- anterior interventricular sulcus
- sulcus located between the left and right ventricles on the anterior surface of the heart
- aortic valve
- (also, aortic semilunar valve) valve located at the base of the aorta
- atrioventricular septum
- cardiac septum located between the atria and ventricles; atrioventricular valves are located here
- atrioventricular valves
- one-way valves located between the atria and ventricles; the valve on the right is called the tricuspid valve, and the one on the left is the mitral or bicuspid valve
- atrium
- (plural = atria) upper or receiving chamber of the heart that pumps blood into the lower chambers just prior to their contraction; the right atrium receives blood from the systemic circuit that flows into the right ventricle; the left atrium receives blood from the pulmonary circuit that flows into the left ventricle
- auricle
- extension of an atrium visible on the superior surface of the heart
- bicuspid valve
- (also, mitral valve or left atrioventricular valve) valve located between the left atrium and ventricle; consists of two flaps of tissue
- cardiac notch
- depression in the medial surface of the inferior lobe of the left lung where the apex of the heart is located
- cardiac skeleton
- (also, skeleton of the heart) reinforced connective tissue located within the atrioventricular septum; includes four rings that surround the openings between the atria and ventricles, and the openings to the pulmonary trunk and aorta; the point of attachment for the heart valves
- cardiomyocyte
- muscle cell of the heart
- chordae tendineae
- string-like extensions of tough connective tissue that extend from the flaps of the atrioventricular valves to the papillary muscles
- circumflex artery
- branch of the left coronary artery that follows coronary sulcus
- coronary arteries
- branches of the ascending aorta that supply blood to the heart; the left coronary artery feeds the left side of the heart, the left atrium and ventricle, and the interventricular septum; the right coronary artery feeds the right atrium, portions of both ventricles, and the heart conduction system
- coronary sinus
- large, thin-walled vein on the posterior surface of the heart that lies within the atrioventricular sulcus and drains the heart myocardium directly into the right atrium
- coronary sulcus
- sulcus that marks the boundary between the atria and ventricles
- coronary veins
- vessels that drain the heart and generally parallel the large surface arteries
- endocardium
- innermost layer of the heart lining the heart chambers and heart valves; composed of endothelium reinforced with a thin layer of connective tissue that binds to the myocardium
- endothelium
- layer of smooth, simple squamous epithelium that lines the endocardium and blood vessels
- epicardial coronary arteries
- surface arteries of the heart that generally follow the sulci
- epicardium
- innermost layer of the serous pericardium and the outermost layer of the heart wall
- foramen ovale
- opening in the fetal heart that allows blood to flow directly from the right atrium to the left atrium, bypassing the fetal pulmonary circuit
- fossa ovalis
- oval-shaped depression in the interatrial septum that marks the former location of the foramen ovale
- great cardiac vein
- vessel that follows the interventricular sulcus on the anterior surface of the heart and flows along the coronary sulcus into the coronary sinus on the posterior surface; parallels the anterior interventricular artery and drains the areas supplied by this vessel
- hypertrophic cardiomyopathy
- pathological enlargement of the heart, generally for no known reason
- inferior vena cava
- large systemic vein that returns blood to the heart from the inferior portion of the body
- interatrial septum
- cardiac septum located between the two atria; contains the fossa ovalis after birth
- interventricular septum
- cardiac septum located between the two ventricles
- left atrioventricular valve
- (also, mitral valve or bicuspid valve) valve located between the left atrium and ventricle; consists of two flaps of tissue
- marginal arteries
- branches of the right coronary artery that supply blood to the superficial portions of the right ventricle
- mesothelium
- simple squamous epithelial portion of serous membranes, such as the superficial portion of the epicardium (the visceral pericardium) and the deepest portion of the pericardium (the parietal pericardium)
- middle cardiac vein
- vessel that parallels and drains the areas supplied by the posterior interventricular artery; drains into the great cardiac vein
- mitral valve
- (also, left atrioventricular valve or bicuspid valve) valve located between the left atrium and ventricle; consists of two flaps of tissue
- moderator band
- band of myocardium covered by endocardium that arises from the inferior portion of the interventricular septum in the right ventricle and crosses to the anterior papillary muscle; contains conductile fibers that carry electrical signals followed by contraction of the heart
- myocardium
- thickest layer of the heart composed of cardiac muscle cells built upon a framework of primarily collagenous fibers and blood vessels that supply it and the nervous fibers that help to regulate it
- papillary muscle
- extension of the myocardium in the ventricles to which the chordae tendineae attach
- pectinate muscles
- muscular ridges seen on the anterior surface of the right atrium
- pericardial cavity
- cavity surrounding the heart filled with a lubricating serous fluid that reduces friction as the heart contracts
- pericardial sac
- (also, pericardium) membrane that separates the heart from other mediastinal structures; consists of two distinct, fused sublayers: the fibrous pericardium and the parietal pericardium
- pericardium
- (also, pericardial sac) membrane that separates the heart from other mediastinal structures; consists of two distinct, fused sublayers: the fibrous pericardium and the parietal pericardium
- posterior cardiac vein
- vessel that parallels and drains the areas supplied by the marginal artery branch of the circumflex artery; drains into the great cardiac vein
- posterior interventricular artery
- (also, posterior descending artery) branch of the right coronary artery that runs along the posterior portion of the interventricular sulcus toward the apex of the heart and gives rise to branches that supply the interventricular septum and portions of both ventricles
- posterior interventricular sulcus
- sulcus located between the left and right ventricles on the anterior surface of the heart
- pulmonary arteries
- left and right branches of the pulmonary trunk that carry deoxygenated blood from the heart to each of the lungs
- pulmonary capillaries
- capillaries surrounding the alveoli of the lungs where gas exchange occurs: carbon dioxide exits the blood and oxygen enters
- pulmonary circuit
- blood flow to and from the lungs
- pulmonary trunk
- large arterial vessel that carries blood ejected from the right ventricle; divides into the left and right pulmonary arteries
- pulmonary valve
- (also, pulmonary semilunar valve, the pulmonic valve, or the right semilunar valve) valve at the base of the pulmonary trunk that prevents backflow of blood into the right ventricle; consists of three flaps
- pulmonary veins
- veins that carry highly oxygenated blood into the left atrium, which pumps the blood into the left ventricle, which in turn pumps oxygenated blood into the aorta and to the many branches of the systemic circuit
- right atrioventricular valve
- (also, tricuspid valve) valve located between the right atrium and ventricle; consists of three flaps of tissue
- semilunar valves
- valves located at the base of the pulmonary trunk and at the base of the aorta
- septum
- (plural = septa) walls or partitions that divide the heart into chambers
- septum primum
- flap of tissue in the fetus that covers the foramen ovale within a few seconds after birth</dd
- small cardiac vein
- parallels the right coronary artery and drains blood from the posterior surfaces of the right atrium and ventricle; drains into the great cardiac vein
- sulcus
- (plural = sulci) fat-filled groove visible on the surface of the heart; coronary vessels are also located in these areas
- superior vena cava
- large systemic vein that returns blood to the heart from the superior portion of the body
- systemic circuit
- blood flow to and from virtually all of the tissues of the body
- trabeculae carneae
- ridges of muscle covered by endocardium located in the ventricles
- tricuspid valve
- term used most often in clinical settings for the right atrioventricular valve
- valve
- in the cardiovascular system, a specialized structure located within the heart or vessels that ensures one-way flow of blood
- ventricle
- one of the primary pumping chambers of the heart located in the lower portion of the heart; the left ventricle is the major pumping chamber on the lower left side of the heart that ejects blood into the systemic circuit via the aorta and receives blood from the left atrium; the right ventricle is the major pumping chamber on the lower right side of the heart that ejects blood into the pulmonary circuit via the pulmonary trunk and receives blood from the right atrium
Solutions
Answers for Critical Thinking Questions
- When the ventricles contract and pressure begins to rise in the ventricles, there is an initial tendency for blood to flow back (regurgitate) to the atria. However, the papillary muscles also contract, placing tension on the chordae tendineae and holding the atrioventricular valves (tricuspid and mitral) in place to prevent the valves from prolapsing and being forced back into the atria. The semilunar valves (pulmonary and aortic) lack chordae tendineae and papillary muscles, but do not face the same pressure gradients as do the atrioventricular valves. As the ventricles relax and pressure drops within the ventricles, there is a tendency for the blood to flow backward. However, the valves, consisting of reinforced endothelium and connective tissue, fill with blood and seal off the opening preventing the return of blood.
- The pulmonary circuit consists of blood flowing to and from the lungs, whereas the systemic circuit carries blood to and from the entire body. The systemic circuit is far more extensive, consisting of far more vessels and offers much greater resistance to the flow of blood, so the heart must generate a higher pressure to overcome this resistance. This can be seen in the thickness of the myocardium in the ventricles.
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax, are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.



