25.5 Physiology of Urine Formation: Tubular Reabsorption and Secretion
Learning Objectives
By the end of this section, you will be able to:
- List specific transport mechanisms occurring in different parts of the nephron, including active transport, osmosis, facilitated diffusion, and passive electrochemical gradients
- List the different membrane proteins of the nephron, including channels, transporters, and ATPase pumps
- Compare and contrast passive and active tubular reabsorption
- Explain why the differential permeability or impermeability of specific sections of the nephron tubules is necessary for urine formation
- Describe how and where water, organic compounds, and ions are reabsorbed in the nephron
- Explain the role of the loop of Henle, the vasa recta, and the countercurrent multiplication mechanisms in the concentration of urine
- List the locations in the nephron where tubular secretion occurs
- Describe how, where, and what substances are secreted by the nephron.
With up to 180 liters per day passing through the nephrons of the kidney, it is quite obvious that most of that fluid and its contents must be reabsorbed. Recall that substances that need to be removd from the body but were not yet filtered, can be secreted. This reabsorption occurs in the PCT, loop of Henle, DCT, and the collecting ducts while the majority of secretion occurs in the PCT and DCT (Table 25.5 and Figure 25.5.1). Various portions of the nephron differ in their capacity to reabsorb water and specific solutes. While much of the reabsorption and secretion occur passively based on concentration gradients, the amount of water that is reabsorbed or lost is tightly regulated. This control is exerted directly by ADH and aldosterone, and indirectly by renin. Most water is recovered in the PCT, loop of Henle, and DCT. About 10 percent (about 18 L) reaches the collecting ducts. The collecting ducts, under the influence of ADH, can recover almost all of the water passing through them, in cases of dehydration, or almost none of the water, in cases of over-hydration.
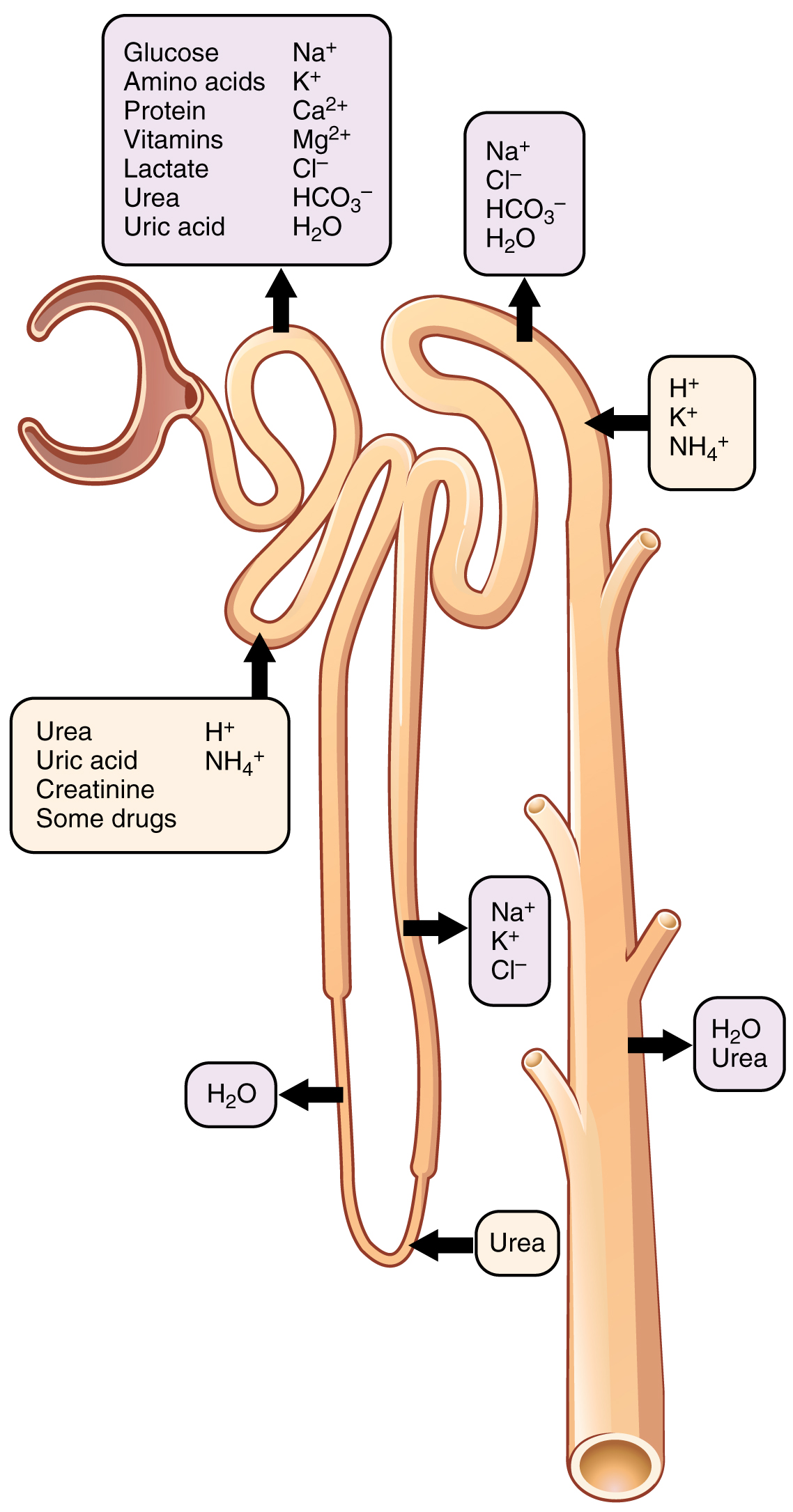
| Substances Secreted or Reabsorbed in the Nephron and Their Locations (Table 25.5) | ||||
|---|---|---|---|---|
| Substance | PCT | Loop of Henle | DCT | Collecting ducts |
| Glucose | Almost 100% reabsorbed; secondary active transport with Na+ | |||
| Oligopeptides, proteins, amino acids | Almost 100% reabsorbed; symport with Na+ | |||
| Vitamins | Reabsorbed | |||
| Lactate | Reabsorbed | |||
| Creatinine | Secreted | |||
| Urea | 50% reabsorbed by diffusion; also secreted | Secretion, diffusion in descending limb | Reabsorption in medullary collecting ducts; diffusion | |
| Sodium | 65% actively reabsorbed | 25 percent reabsorbed in thick ascending limb; active transport | 5 percent reabsorbed; active | 5 percent reabsorbed, stimulated by aldosterone; active |
| Chloride | Reabsorbed, symport with Na+, diffusion | Reabsorbed in thin and thick ascending limb; diffusion in ascending limb | Reabsorbed; diffusion | Reabsorbed; symport |
| Water | 67% reabsorbed osmotically with solutes | 15 percent reabsorbed in descending limb; osmosis | 8 percent reabsorbed if ADH; osmosis | Variable amounts reabsorbed, controlled by ADH, osmosis |
| Bicarbonate | 80–90% symport reabsorption with Na+ | Reabsorbed, symport with Na+ and antiport with Cl–; in ascending limb | Reabsorbed antiport with Cl– | |
| H+ | Secreted; diffusion | Secreted; active | Secreted; active | |
| NH4+ | Secreted; diffusion | Secreted; diffusion | Secreted; diffusion | |
| HCO3– | Reabsorbed; diffusion | Reabsorbed; diffusion in ascending limb | Reabsorbed; diffusion | Reabsorbed; antiport with Na+ |
| Some drugs | Secreted | Secreted; active | Secreted; active | |
| Potassium | 65% reabsorbed; diffusion | 20 percent reabsorbed in thick ascending limb; symport | Secreted; active | Secretion controlled by aldosterone; active |
| Calcium | Reabsorbed; diffusion | Reabsorbed in thick ascending limb; diffusion | Reabsorbed if parathyroid hormone present; active | |
| Magnesium | Reabsorbed; diffusion | Reabsorbed in thick ascending limb; diffusion | Reabsorbed | |
| Phosphate | 85% reabsorbed, inhibited by parathyroid hormone, diffusion | Reabsorbed; diffusion | ||
Mechanisms of Recovery
Mechanisms by which substances move across membranes for reabsorption or secretion include active transport, diffusion, facilitated diffusion, secondary active transport, and osmosis. These were discussed in an earlier chapter, and you may wish to review them.
Active transport utilizes energy, usually the energy found in a phosphate bond of ATP, to move a substance across a membrane from a low to a high concentration. It is very specific and must have an appropriately shaped receptor for the substance to be transported. An example would be the active transport of Na+ out of a cell and K+ into a cell by the Na+/K+ pump. Both ions are moved in opposite directions from a lower to a higher concentration.
Simple diffusion moves a substance from a higher to a lower concentration down its concentration gradient. It requires no energy and only needs to be soluble.
Facilitated diffusion is similar to diffusion in that it moves a substance down its concentration gradient. The difference is that it requires specific membrane receptors or channel proteins for movement. The movement of glucose and, in certain situations, Na+ ions, is an example of facilitated diffusion. In some cases of facilitated diffusion, two different substances share the same channel protein port; these mechanisms are described by the terms symport and antiport.
Symport mechanisms move two or more substances in the same direction at the same time, whereas antiport mechanisms move two or more substances in opposite directions across the cell membrane. Both mechanisms may utilize concentration gradients maintained by ATP pumps. This is a mechanism described by the term “secondary active transport.” For example, a Na+ ATPase pump on the basilar membrane of a cell may constantly pump Na+ out of a cell, maintaining a strong electrochemical gradient. On the opposite (apical) surface, a Na+/glucose symport protein channel assists both Na+ and glucose into the cell as Na+ moves down the concentration gradient created by the basilar Na+ ATPase pumps. The glucose molecule then diffuses across the basal membrane by facilitated diffusion into the interstitial space and from there into peritubular capillaries.
Most of the Ca++, Na+, glucose, and amino acids must be reabsorbed by the nephron to maintain homeostatic plasma concentrations. Other substances, such as urea, K+, ammonia (NH3), creatinine, and some drugs are secreted into the filtrate as waste products. Acid–base balance is maintained through actions of the lungs and kidneys: The lungs rid the body of H+, whereas the kidneys secrete or reabsorb H+ and HCO3– (Table 25.6). In the case of urea, about 50 percent is passively reabsorbed by the PCT. More is recovered by in the collecting ducts as needed. ADH induces the insertion of urea transporters and aquaporin channel proteins.
| Substances Filtered and Reabsorbed by the Kidney per 24 Hours (Table 25.6) | |||
|---|---|---|---|
| Substance | Amount filtered (grams) | Amount reabsorbed (grams) | Amount in urine (grams) |
| Water | 180 L | 179 L | 1 L |
| Proteins | 10–20 | 10–20 | 0 |
| Chlorine | 630 | 625 | 5 |
| Sodium | 540 | 537 | 3 |
| Bicarbonate | 300 | 299.7 | 0.3 |
| Glucose | 180 | 180 | 0 |
| Urea | 53 | 28 | 25 |
| Potassium | 28 | 24 | 4 |
| Uric acid | 8.5 | 7.7 | 0.8 |
| Creatinine | 1.4 | 0 | 1.4 |
Reabsorption in the Proximal Convoluted Tubule
The renal corpuscle filters the blood to create a filtrate that still contains many important molecules that the body needs to reclaim. The PCT reclaims more of these than any other portion of the nephron. The cells of the PCT have two surfaces: apical faces the lumen of the tubule and is in contact with the filtrate. The basal surface of the PCT cell faces the interstitial space near the peritubular capillary. Sodium is actively pumped by the PCT cells into the interstitial space and diffuses down its concentration gradient into the peritubular capillary. As it does so, water follows passively by osmosis. This is called obligatory water reabsorption, because water is “obliged” to follow the Na+ (Figure 25.5.2). Filtered amino acids and glucose move with sodium using specific membrane transport proteins (symports), accounting for 100% of reabsorption of these molecules in healthy individuals. Both glucose and Na+ bind simultaneously to the same symport proteins on the apical surface of the cell to be transported in the same direction, toward the interstitial space. Sodium moves down its electrochemical and concentration gradient into the cell and takes glucose with it. Na+ is then actively pumped out of the cell at the basal surface of the cell into the interstitial space. Glucose leaves the cell to enter the interstitial space by facilitated diffusion. The energy to move glucose comes from the Na+/K+ ATPase that pumps Na+ out of the cell on the basal surface. The numbers and particular types of pumps and channels vary between the apical and basilar surfaces (Table 25.7) as well as the directionality of movement. Some molecules do not require cellular transport proteins but instead move between adjacent cell membranes (paracellular) across the tubule and back into the blood.
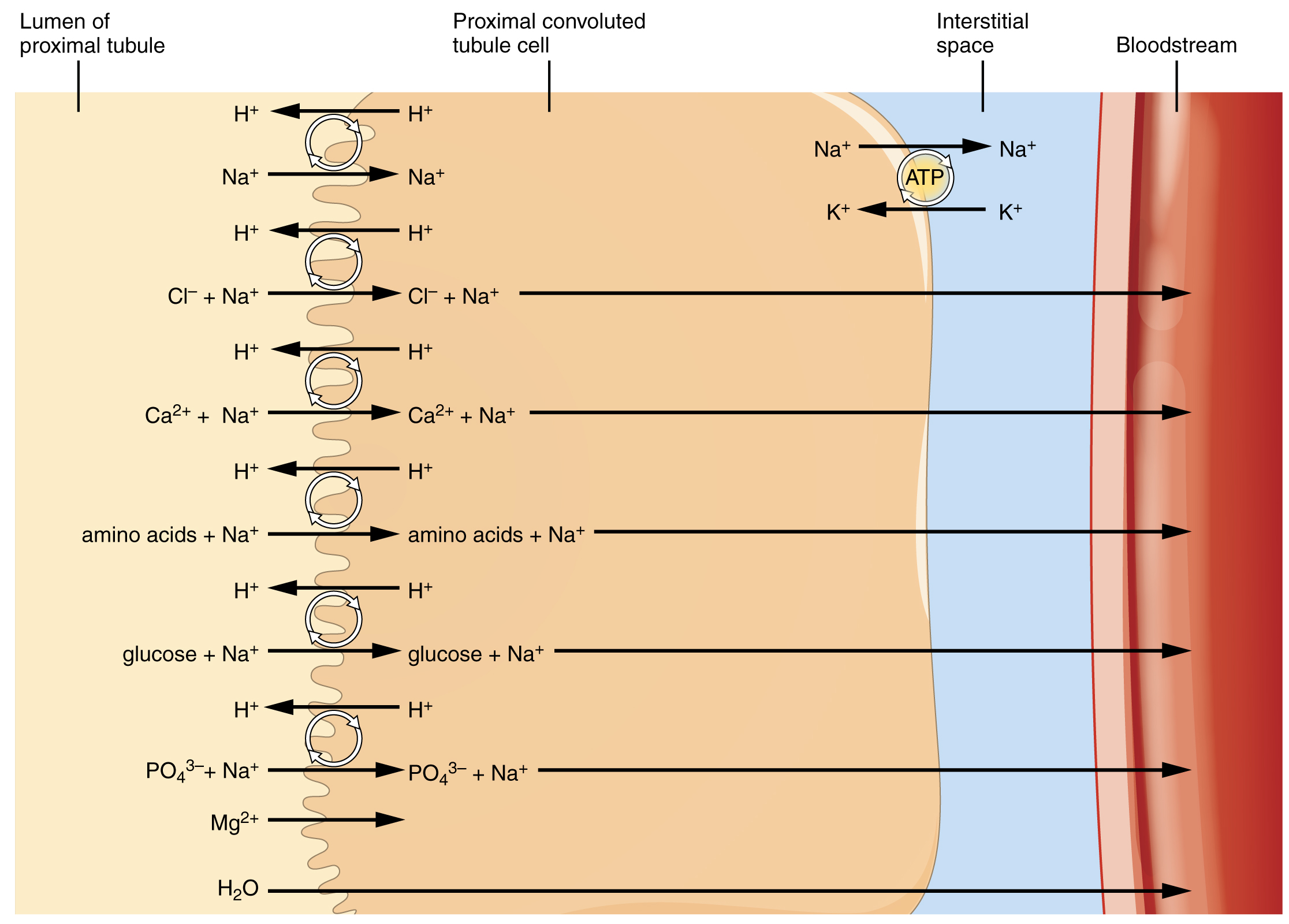
About 67 percent of the water, Na+, and K+ entering the nephron is reabsorbed in the PCT and returned to the circulation. Almost 100 percent of glucose, amino acids, and other organic substances such as vitamins are normally recovered here. Some glucose may appear in the urine if circulating glucose levels are high enough that all the glucose transporters in the PCT are saturated, so that their capacity to move glucose is exceeded (transport maximum, or Tm) like that seen with diabetes mellitus. Fifty percent of Cl– and variable quantities of HCO3–, Ca++, Mg++, and HPO42− are also recovered in the PCT. The significant recovery of solutes from the PCT lumen to the interstitial space creates an osmotic gradient that promotes water recovery.
| Reabsorption of Major Solutes by the PCT (Table 25.7) | |
|---|---|
| Basal membrane | Apical membrane |
| Active transport | Symport with Na+ |
| Na+ (exchange for K+) | K+ |
| Facilitated diffusion | Cl– |
| K+ | Ca++ |
| Cl– | Mg++ |
| Ca++ | HCO3– |
| HCO3– | PO43− |
| PO43− | Amino acids |
| Amino acids | Glucose |
| Glucose | Fructose |
| Fructose | Galactose |
| Galactose | Lactate |
| Lactate | Succinate |
| Succinate | Citrate |
| Citrate | Diffusion between nephron cells |
| K+ | |
| Ca++ | |
| Mg++ | |
About 67 percent of the water, Na+, and K+ entering the nephron is reabsorbed in the PCT and returned to the circulation. Almost 100 percent of glucose, amino acids, and other organic substances such as vitamins are normally recovered here. Some glucose may appear in the urine if circulating glucose levels are high enough that all the glucose transporters in the PCT are saturated, so that their capacity to move glucose is exceeded (transport maximum, or Tm). In men, the maximum amount of glucose that can be recovered is about 375 mg/min, whereas in women, it is about 300 mg/min. This recovery rate translates to an arterial concentration of about 200 mg/dL. Though an exceptionally high sugar intake might cause sugar to appear briefly in the urine, the appearance of glycosuria usually points to type I or II diabetes mellitus. The transport of glucose from the lumen of the PCT to the interstitial space is similar to the way it is absorbed by the small intestine. Both glucose and Na+ bind simultaneously to the same symport proteins on the apical surface of the cell to be transported in the same direction, toward the interstitial space. Sodium moves down its electrochemical and concentration gradient into the cell and takes glucose with it. Na+ is then actively pumped out of the cell at the basal surface of the cell into the interstitial space. Glucose leaves the cell to enter the interstitial space by facilitated diffusion. The energy to move glucose comes from the Na+/K+ ATPase that pumps Na+ out of the cell on the basal surface. Fifty percent of Cl– and variable quantities of Ca++, Mg++, and HPO42− are also recovered in the PCT.
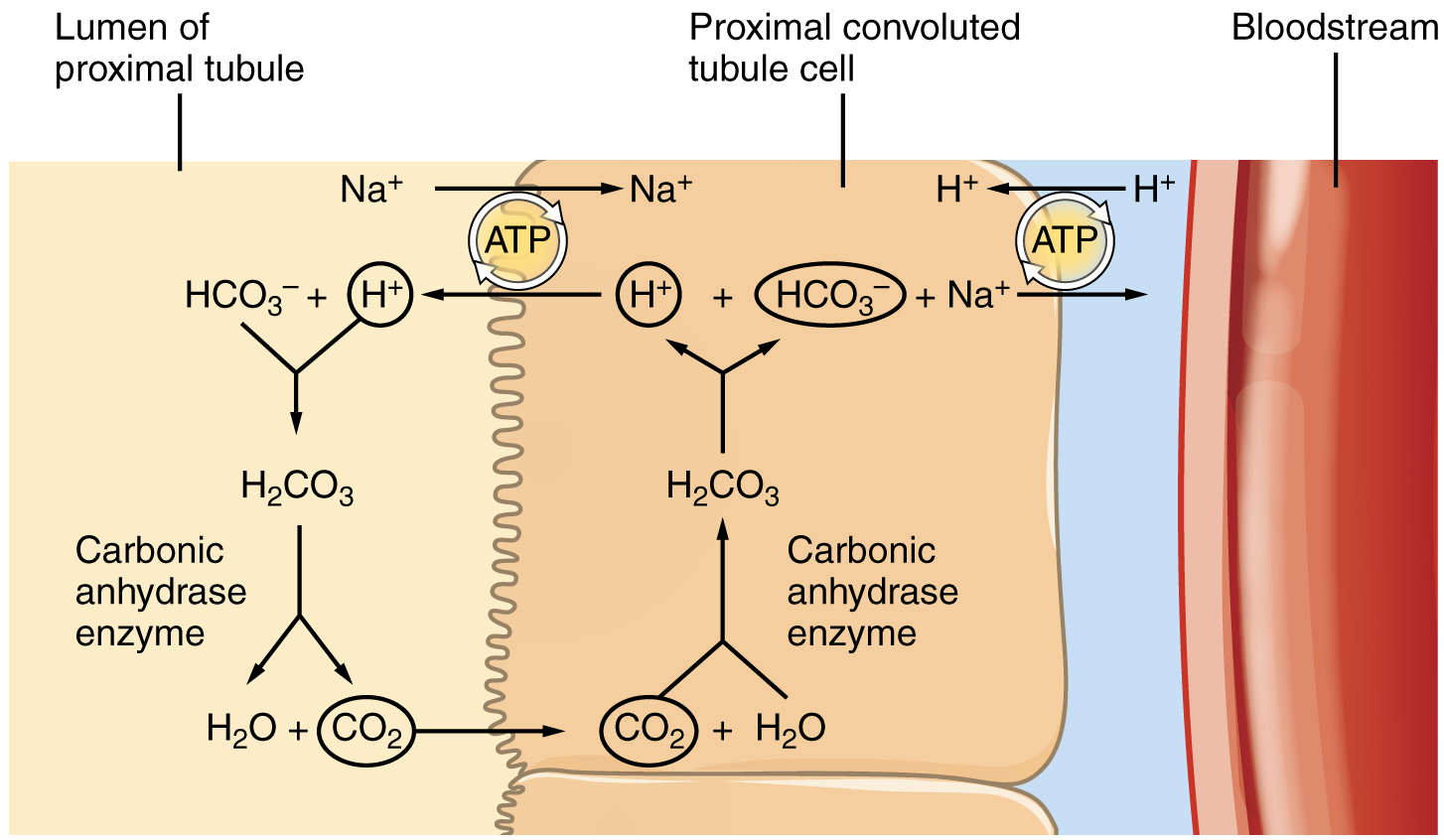
Recovery of bicarbonate (HCO3–) is vital to the maintenance of acid–base balance, since it is a very powerful and fast-acting buffer. An important enzyme is used to catalyze this mechanism: carbonic anhydrase (CA). This same enzyme and reaction is used in red blood cells in the transportation of CO2, in the stomach to produce hydrochloric acid, and in the pancreas to produce HCO3– to buffer acidic chyme from the stomach. In the kidney, most of the CA is located within the cell, but a small amount is bound to the brush border of the membrane on the apical surface of the cell. In the lumen of the PCT, HCO3– combines with hydrogen ions to form carbonic acid (H2CO3). This is enzymatically catalyzed into CO2 and water, which diffuse across the apical membrane into the cell. Water can move osmotically across the lipid bilayer membrane due to the presence of aquaporin water channels. Inside the cell, the reverse reaction occurs to produce bicarbonate ions (HCO3–). These bicarbonate ions are cotransported with Na+ across the basal membrane to the interstitial space around the PCT (Figure 25.5.3). At the same time this is occurring, a Na+/H+ antiporter excretes H+ into the lumen, while it recovers Na+. Note how the hydrogen ion is recycled so that bicarbonate can be recovered. Also, note that a Na+ gradient is created by the Na+/K+ pump.
HCO3−+ H+ ↔ H2CO3 ↔ CO2 + H2O
Reabsorption in the Loop of Henle
The loop of Henle consists of two sections: thick and thin descending and thin and thick ascending sections. The loops of cortical nephrons do not extend into the renal medulla very far, if at all. Juxtamedullary nephrons have loops that extend variable distances, some very deep into the medulla. The descending and ascending portions of the loop are highly specialized to enable recovery of the remaining Na+ and water that were filtered by the glomerulus but not yet reabsorbed. As the filtrate moves through the loop, its osmolarity will change from iso-osmotic with blood (about 278–300 mOsmol/kg) to first a very hypertonic solution of about 1200 mOsmol/kg and then a very hypotonic solution of about 100 mOsmol/kg. These changes are accomplished by osmosis in the descending limb and active transport of salt in the ascending limb. Solutes and water recovered from these loops are returned to the circulation by way of the peritubular capillaries (cortical nephron) or vasa recta (juxtamedullary nephron).
Descending Loop
The majority of the descending loop is comprised of simple squamous epithelial cells; to simplify the function of the loop, this discussion focuses on these cells. These membranes have permanent aquaporin channel proteins that allow unrestricted movement of water from the descending loop into the surrounding interstitium as the osmolarity of the filtrate increases from about 300 mOsmol/kg to about 1200 mOsmol/kg. This increase results in reabsorption of up to 15 percent of the water entering the nephron. Modest amounts of urea, Na+, and other ions are also recovered here.
Most of the solutes that were filtered in the glomerulus have now been recovered along with a majority of water, about 82 percent. As the filtrate enters the ascending loop, major adjustments will be made to the concentration of solutes to create what you perceive as urine.
Ascending Loop
The ascending loop is made of thin and thick portions. The thick portion is lined with simple cuboidal epithelium without a brush border. It is relatively impermeable to water due to the absence of aquaporin proteins. However, ions are actively pumped out of the loop by large quantities of the Na+/K+ ATPase pump coupled with specific ion channels. The Na+/K+ ATPase pumps in the basal membrane create an electrochemical gradient, allowing reabsorption of Cl– by Na+/Cl– symporters in the apical membrane. At the same time that Na+ is actively pumped from the basal side of the cell into the interstitial fluid, Cl– follows the Na+ from the lumen into the interstitial fluid by a paracellular route between cells through leaky tight junctions. Most of the K+ that enters the cell via symporters returns to the lumen (down its concentration gradient) through leaky channels in the apical membrane. This action creates a negative charge in the interstitial fluid which attracts cations (Na+, K+, Ca++, and Mg++) from the lumen via a paracellular route to the interstitial space and vasa recta. These actions have two significant effects: 1) Removal of Na+ while retaining water leads to a hypotonic filtrate flowing to the DCT and 2) pumping Na+ into the interstitial space creates a hyperosmotic interstitial fluid environment in the kidney medulla.
Reabsorption in the Distal Convoluted Tubule and Collecting Ducts
Approximately 80 percent of filtered water has been recovered by the time the dilute filtrate enters the DCT. The DCT will recover another 10–15 percent before the filtrate enters the collecting ducts. Under hormonal action, additional water and solutes can be reabsorbed into the peritubular capillaries and returned to the circulation.
Cells of the DCT also recover Ca++ from the filtrate. Receptors for parathyroid hormone (PTH) are found in DCT cells and when bound to PTH, induce the insertion of calcium channels on their luminal surface. The channels enhance Ca++ recovery from the forming urine. In addition, as Na+ is pumped out of the cell, the resulting electrochemical gradient attracts Ca++ into the cell. Finally, calcitriol (1,25 dihydroxyvitamin D, the active form of vitamin D) is very important for calcium recovery. It induces the production of calcium-binding proteins that transport Ca++ into the cell. These binding proteins are also important for the movement of calcium inside the cell and aid in exocytosis of calcium across the basolateral membrane. Any Ca++ not reabsorbed at this point is lost in the urine.
Tubular Secretion
Tubular secretion occurs mostly in the PCT and DCT where unfiltered substances are moved from the peritubular capillary into the lumen of the tubule. Secretion usually removes substances that are too large to be filtered (ex: antibiotics, toxins) or those that are in excess in the blood (ex: H+, K+).
Tubular Reabsorption and Secretion to Control pH
In the next chapter we will discuss how the kidney controls acid-base balance, but it important to understand the reabsorption and secretion mechanisms that the kidney uses to maintain this balance.
The amino acid glutamine can be deaminated by the kidney. As NH2 from the amino acid is converted into NH3 and pumped into the lumen of the PCT, Na+ and HCO3– are excreted into the interstitial fluid of the renal pyramid via a symport mechanism. When this process occurs in the cells of the PCT, the added benefit is a net loss of a hydrogen ion (complexed to ammonia to form the weak acid NH4+) in the urine and a gain of a bicarbonate ion (HCO3–) in the blood. Ammonia and bicarbonate are exchanged in a one-to-one ratio. This exchange is yet another means by which the body can buffer and excrete acid.
Solutes move across the membranes of the cells of the collecting ducts, which contain two distinct cell types, principal cells and intercalated cells. A principal cell possesses channels for the recovery or loss of sodium and potassium. An intercalated cell secretes or absorbs acid or bicarbonate. As in other portions of the nephron, there is an array of micromachines (pumps and channels) on display in the membranes of these cells.
The DCT and collecting ducts contain two distinct cell types, principal cells and intercalated cells. Principal cells function to control sodium and potassium balance. Intercalated cells play significant roles in regulating blood pH. Intercalated cells reabsorb K+ and HCO3– while secreting H+. This function lowers the acidity of the plasma while increasing the acidity of the urine.
Recovery of bicarbonate (HCO3–) is vital to the maintenance of acid–base balance, since it is a very powerful and fast-acting buffer. An important enzyme is used to catalyze this mechanism: carbonic anhydrase (CA). This same enzyme and reaction is used in red blood cells in the transportation of CO2, in the stomach to produce hydrochloric acid, and in the pancreas to produce HCO3– to buffer acidic chyme from the stomach. In the kidney, most of the CA is located within the cell, but a small amount is bound to the brush border of the membrane on the apical surface of the cell. In the lumen of the PCT, HCO3– combines with hydrogen ions to form carbonic acid (H2CO3). This is enzymatically catalyzed into CO2 and water, which diffuse across the apical membrane into the cell. Water can move osmotically across the lipid bilayer membrane due to the presence of aquaporin water channels. Inside the cell, the reverse reaction occurs to produce bicarbonate ions (HCO3–). These bicarbonate ions are cotransported with Na+ across the basal membrane to the interstitial space around the PCT (Figure 25.5.4). At the same time this is occurring, a Na+/H+ antiporter excretes H+ into the lumen, while it recovers Na+. Note how the hydrogen ion is recycled so that bicarbonate can be recovered. Also, note that a Na+ gradient is created by the Na+/K+ pump.

Chapter Review
The kidney regulates water recovery and blood pressure by producing the enzyme renin. It is renin that starts a series of reactions, leading to the production of the vasoconstrictor angiotensin II and the salt-retaining steroid aldosterone. Water recovery is also powerfully and directly influenced by the hormone ADH. Even so, it only influences the last 10 percent of water available for recovery after filtration at the glomerulus, because 90 percent of water is recovered before reaching the collecting ducts. Depending on the body’s fluid status at any given time, the collecting ducts can recover none or almost all of the water reaching them.
Mechanisms of solute recovery include active transport, simple diffusion, and facilitated diffusion. Most filtered substances are reabsorbed. Urea, NH3, creatinine, and some drugs are filtered or secreted as wastes. H+ and HCO3– are secreted or reabsorbed as needed to maintain acid–base balance. Movement of water from the glomerulus is primarily due to pressure, whereas that of peritubular capillaries and vasa recta is due to osmolarity and concentration gradients. The PCT is the most metabolically active part of the nephron and uses a wide array of protein micromachines to maintain homeostasis—symporters, antiporters, and ATPase active transporters—in conjunction with diffusion, both simple and facilitated. Almost 100 percent of glucose, amino acids, and vitamins are recovered in the PCT. Bicarbonate (HCO3–) is recovered using the same enzyme, carbonic anhydrase (CA), found in erythrocytes. The recovery of solutes creates an osmotic gradient to promote the recovery of water. The descending loop of the juxtaglomerular nephrons reaches an osmolarity of up to 1200 mOsmol/kg, promoting the recovery of water. The ascending loop is impervious to water but actively recovers Na+, reducing filtrate osmolarity to 50–100 mOsmol/kg. The descending and ascending loop and vasa recta form a countercurrent multiplier system to increase Na+ concentration in the kidney medulla. The collecting ducts actively pump urea into the medulla, further contributing to the high osmotic environment. The vasa recta recover the solute and water in the medulla, returning them to the circulation. Nearly 90 percent of water is recovered before the forming urine reaches the DCT, which will recover another 10 percent. Calcium recovery in the DCT is influenced by PTH and active vitamin D. In the collecting ducts, ADH stimulates aquaporin channel insertion to increase water recovery and thereby regulate osmolarity of the blood. Aldosterone stimulates Na+ recovery by the collecting duct.
Review Question
Critical Thinking Questions
Glossary
- glycosuria
- presence of glucose in the urine; caused by high blood glucose levels that exceed the ability of the kidneys to reabsorb the glucose; usually the result of untreated or poorly controlled diabetes mellitus
- intercalated cell
- specialized cell of the collecting ducts that secrete or absorb acid or bicarbonate; important in acid–base balance
- leaky tight junctions
- tight junctions in which the sealing strands of proteins between the membranes of adjacent cells are fewer in number and incomplete; allows limited intercellular movement of solvent and solutes
- principal cell
- found in collecting ducts and possess channels for the recovery or loss of sodium and potassium; under the control of aldosterone; also have aquaporin channels under ADH control to regulate recovery of water
Solutions
Answers for Critical Thinking Questions
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax, are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.

