19.4 Cardiac Physiology
Learning Objectives
By the end of this section, you will be able to:
- Define cardiac output and explain how heart rate and stroke volume effect it
- Describe the effect of exercise on cardiac output
- Identify cardiovascular centers and cardiac reflexes that regulate heart function
- Describe factors affecting heart rate and force of contraction
- Explain the connection between preload, contractility, afterload and stroke volume
- Distinguish between positive and negative inotropic factors
- Summarize factors affecting stroke volume, heart rate and cardiac output
The autorhythmicity inherent in cardiac cells keeps the heart beating at a regular pace; however, the heart is regulated by and responds to outside influences as well. Neural and endocrine controls are vital to the regulation of cardiac function. In addition, the heart is sensitive to several environmental factors, including electrolytes.
Resting Cardiac Output
Cardiac output (CO) is a measurement of the amount of blood pumped by each ventricle in one minute. To calculate this value, multiply stroke volume (SV), the amount of blood pumped by each ventricle, by heart rate (HR), in contractions per minute (or beats per minute, bpm). It can be represented mathematically by the following equation:
CO = HR × SV
SV is normally measured using an echocardiogram to record EDV and ESV, and calculating the difference: SV = EDV – ESV. SV can also be measured using a specialized catheter, but this is an invasive procedure and far more dangerous to the patient. A mean SV for a resting 70-kg (150-lb) individual would be approximately 70 mL. This is because typical EDV and ESV values are approximately 120 mL and 50 mL, respectively and 70 mL = 120 mL – 50 mL. Normal range for SV would be 55–100 mL. An average resting HR would be approximately 75 bpm but could range from 60–100 in some individuals. There are several important variables, including size of the heart, physical and mental condition (via hormones and the ANS) of the individual, gender, contractility, duration of contraction, preload or EDV, and afterload or resistance that can affect SV and HR.
Using these numbers, the mean resting CO is 5.25 L/min, with a range of 4.0–8.0 L/min. The CO of 5.25 L/min, was calculated using the following values.
CO L/min = 75 beats/min x 0.070 L/beat (where 0.070 L is equal to 70 mL).
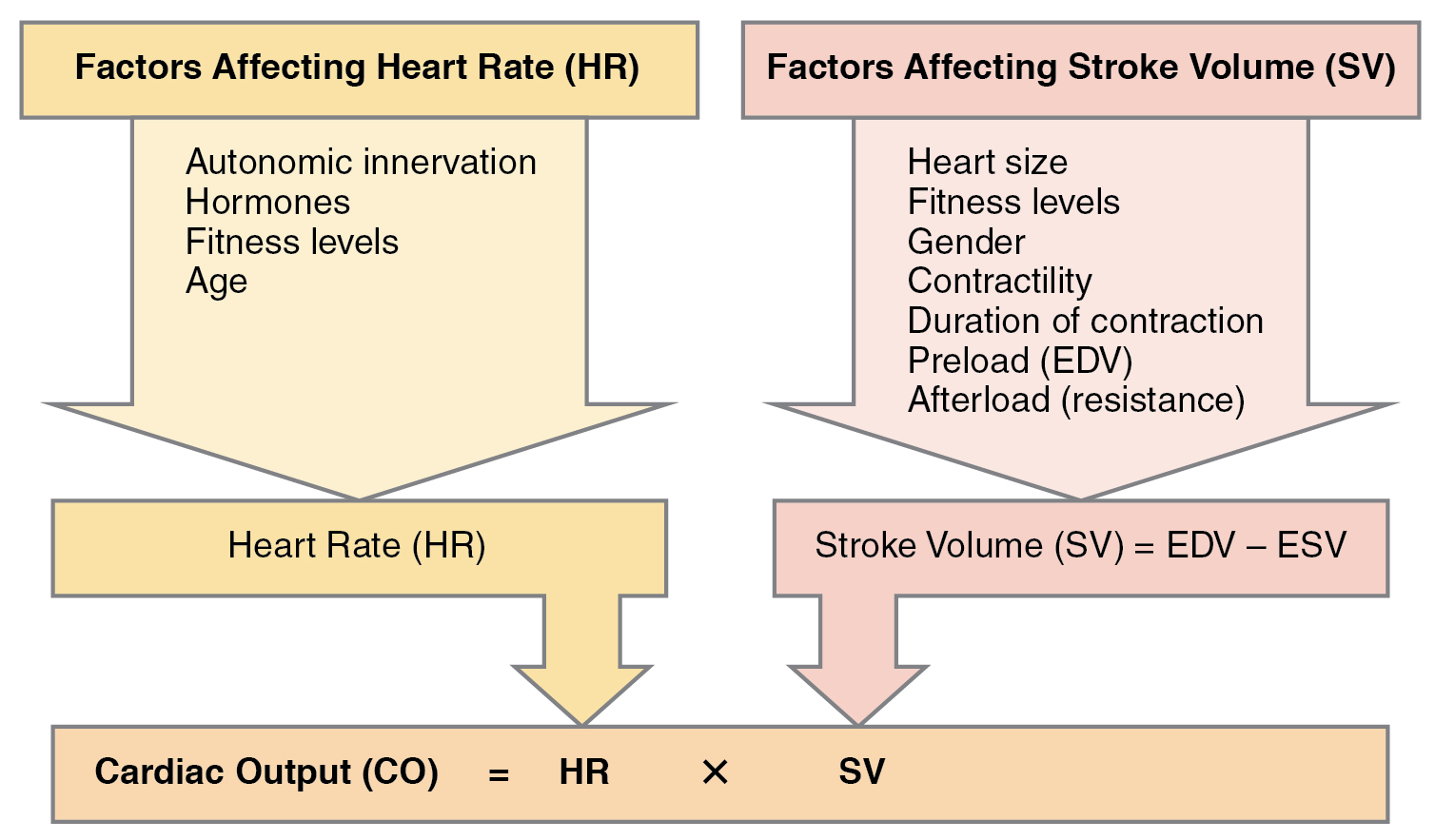
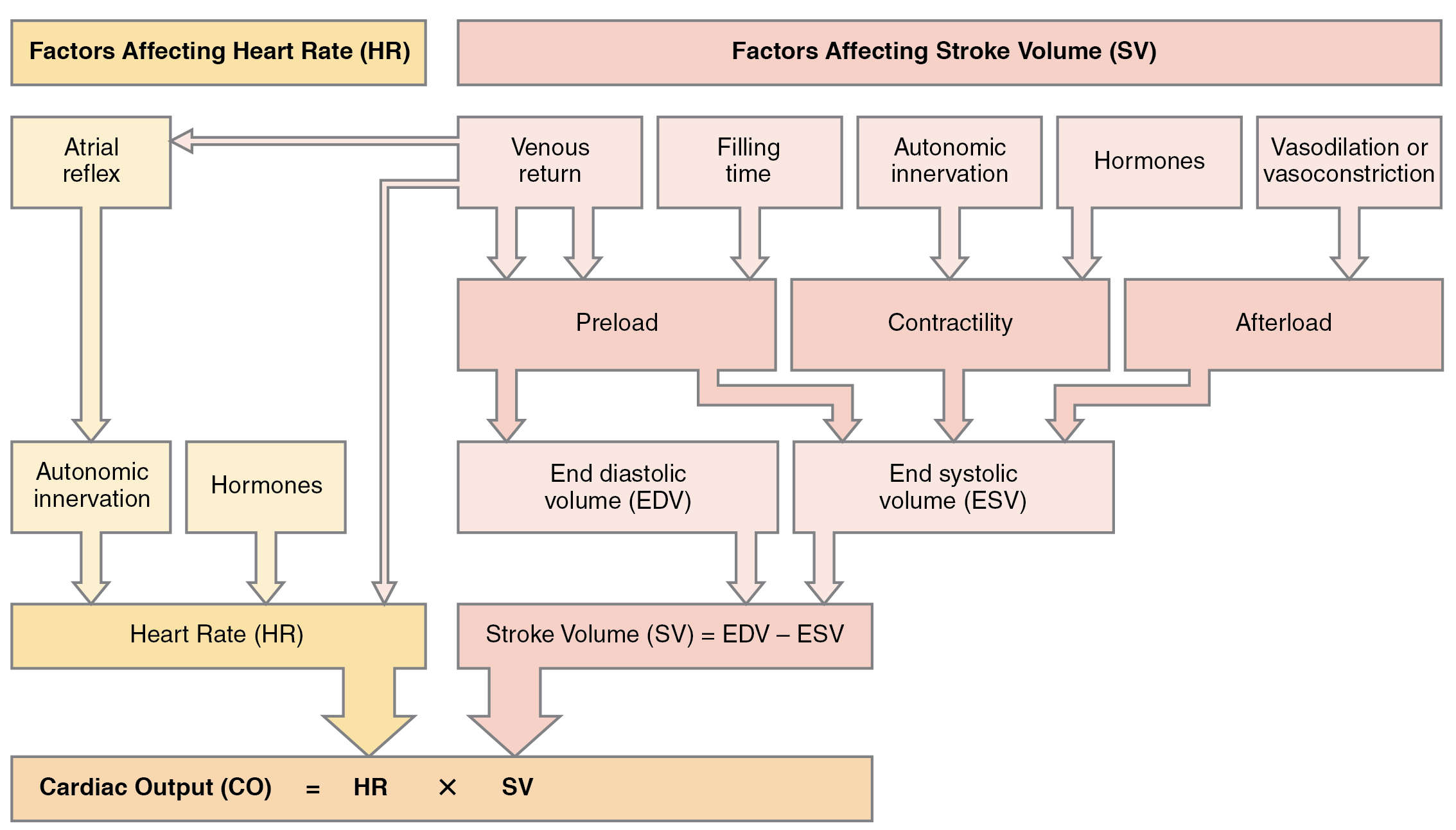
Remember, however, that these numbers refer to CO from each ventricle separately, not the total for the heart. In a healthy heart the CO from each ventricle is the same. CO is influenced by HR and by SV. If SV decreases, CO can be maintained by increasing HR. Factors that influence HR are referred to as chronotropic factors. Chrono- refers to time. Positive chronotropic factors increase HR and negative chronotropic factors decrease HR. HR is influenced by the autonomic nervous system, chemicals, and other factors. The factors influencing CO are summarized in Figure 19.4.1.
SVs are also used to calculate ejection fraction, which is the portion of the blood that is pumped or ejected from the heart with each contraction. To calculate ejection fraction, SV is divided by EDV. Despite the name, the ejection fraction is normally expressed as a percentage. Ejection fractions range from approximately 55–70 percent, with a mean of 58 percent. For example, if the average EDV is 120 mL and the SV is 70 mL, the ejection fraction of 58% is calculated as follows:
Ejection fraction (%) = (70 mL/120 mL) x 100
Exercise and Maximum Cardiac Output
In healthy young individuals, HR may increase to 150 bpm or higher during exercise. SV can also increase from 70 to approximately 130 mL due to increased strength of contraction. This would increase CO to approximately 19.5 L/min, 4–5 times the resting rate. Top cardiovascular athletes can achieve even higher levels. At their peak performance, they may increase resting CO by 7–8 times.
Since the heart is a muscle, exercising it increases its efficiency. The difference between maximum and resting CO is known as the cardiac reserve. It measures the residual capacity of the heart to pump blood.
Heart Rate and its Control
HRs vary considerably, not only with exercise and fitness levels, but also with age. Newborn resting HRs may be 120 bpm. HR gradually decreases until young adulthood and then gradually increases again with age.
Maximum HRs are normally in the range of 200–220 bpm, although there are some extreme cases in which they may reach higher levels. As one ages, the ability to generate maximum rates decreases. This may be estimated by taking the maximal value of 220 bpm and subtracting the individual’s age. So a 40-year-old individual would be expected to reach a maximum rate of approximately 180, and a 60-year-old person would achieve a HR of 160. Refer to the example below.
HRMax = 220 – 60 yr
HRMax = 160 bpm
Bradycardia may be caused by either intrinsic factors or causes external to the heart. While the condition may be inherited, typically it is acquired in older individuals. Intrinsic causes include abnormalities in either the SA or AV node. If the condition is serious, a pacemaker may be required. Other causes include ischemia to the heart muscle or diseases of the heart vessels or valves. External causes include metabolic disorders, pathologies of the endocrine system often involving the thyroid, electrolyte imbalances, neurological disorders including inappropriate autonomic responses, autoimmune pathologies, over-prescription of beta blocker drugs that reduce HR, recreational drug use, or even prolonged bed rest. Treatment relies upon establishing the underlying cause of the disorder and may necessitate supplemental oxygen.
Tachycardia is not normal in a resting patient but may be detected in pregnant women or individuals experiencing extreme stress. In the latter case, it would likely be triggered by stimulation from the limbic system or disorders of the endocrine or autonomic nervous system. In some cases, tachycardia may involve only the atria. Some individuals may remain asymptomatic, but when present, symptoms may include dizziness, shortness of breath, lightheadedness, rapid pulse, heart palpations, chest pain, or fainting (syncope). While tachycardia is defined as a HR above 100 bpm, there is considerable variation among people. Further, the normal resting HRs of children are often above 100 bpm, but this is not considered to be tachycardia Many causes of tachycardia may be benign, but the condition may also be correlated with fever, anemia, hypoxia, hyperthyroidism, hypersecretion of catecholamines, some cardiomyopathies, some disorders of the valves, and acute exposure to radiation. Elevated rates in an exercising or resting patient are normal and expected. Resting rate should always be taken after recovery from exercise. Treatment depends upon the underlying cause but may include medications, implantable cardioverter defibrillators, ablation, or surgery.
Correlation Between Heart Rates and Cardiac Output
Initially, physiological conditions that cause HR to increase also trigger an increase in SV. During exercise, the rate of blood returning to the heart increases. However as the HR rises, there is less time spent in diastole and consequently less time for the ventricles to fill with blood. Even though there is less filling time, SV will initially remain high. However, as HR continues to increase, SV gradually decreases due to decreased filling time. CO will initially stabilize as the increasing HR compensates for the decreasing SV, but at very high rates, CO will eventually decrease as increasing rates are no longer able to compensate for the decreasing SV. Consider this phenomenon in a healthy young individual. Initially, as HR increases from resting to approximately 120 bpm, CO will rise. As HR increases from 120 to 160 bpm, CO remains stable, since the increase in rate is offset by decreasing ventricular filling time and, consequently, SV. As HR continues to rise above 160 bpm, CO actually decreases as SV falls faster than HR increases. So although aerobic exercises are critical to maintain the health of the heart, individuals are cautioned to monitor their HR to ensure they stay within the target heart rate range of between 120 and 160 bpm, so CO is maintained. It is also important to note that the coronary circulation nourishes the heart during diastole so as the HR increases the ability of the coronary circulation to nourish the myocardium decreases. The target HR is loosely defined as the range in which both the heart and lungs receive the maximum benefit from the aerobic workout and is dependent upon age.
Cardiovascular Centers
Nervous control over HR is centralized within the two paired cardiovascular centers of the medulla oblongata (Figure 19.4.2). The cardioaccelerator regions stimulate activity via sympathetic stimulation of the cardioacceleratory nerves, and the cardioinhibitory centers decrease heart activity via parasympathetic stimulation as one component of the vagus nerve, cranial nerve X. Both sympathetic and parasympathetic stimulations flow through a paired complex network of nerve fibers known as the cardiac plexus near the base of the heart. The cardioacceleratory center also sends additional fibers, forming the cardiac nerves via sympathetic ganglia (the cervical ganglia plus superior thoracic ganglia T1–T4) to both the SA and AV nodes to increase heart rate, plus additional fibers to the atrial and ventricular myocardium to increase force of contraction (see section on Contractility). The ventricles are more richly innervated by sympathetic fibers than parasympathetic fibers. During rest, both centers provide slight stimulation to the heart, contributing to autonomic tone. This is a similar concept to tone in skeletal muscles. Normally, vagal stimulation predominates as, left unregulated, the SA node would initiate a sinus rhythm of approximately 100 bpm.
At the nodes sympathetic stimulation causes the release of the neurotransmitter norepinephrine (NE) at the neuromuscular junction of the cardiac nerves. NE binds to the beta-1 receptors and opens chemical- or ligand-gated sodium and calcium ion channels, allowing an influx of positively charged ions. NE shortens the repolarization period, thus speeding the rate of depolarization and contraction, which results in an increase in HR. Some cardiac medications (for example, beta blockers) work by blocking these receptors, thereby slowing HR and are one possible treatment for hypertension. Overprescription of these drugs may lead to bradycardia and even stoppage of the heart.
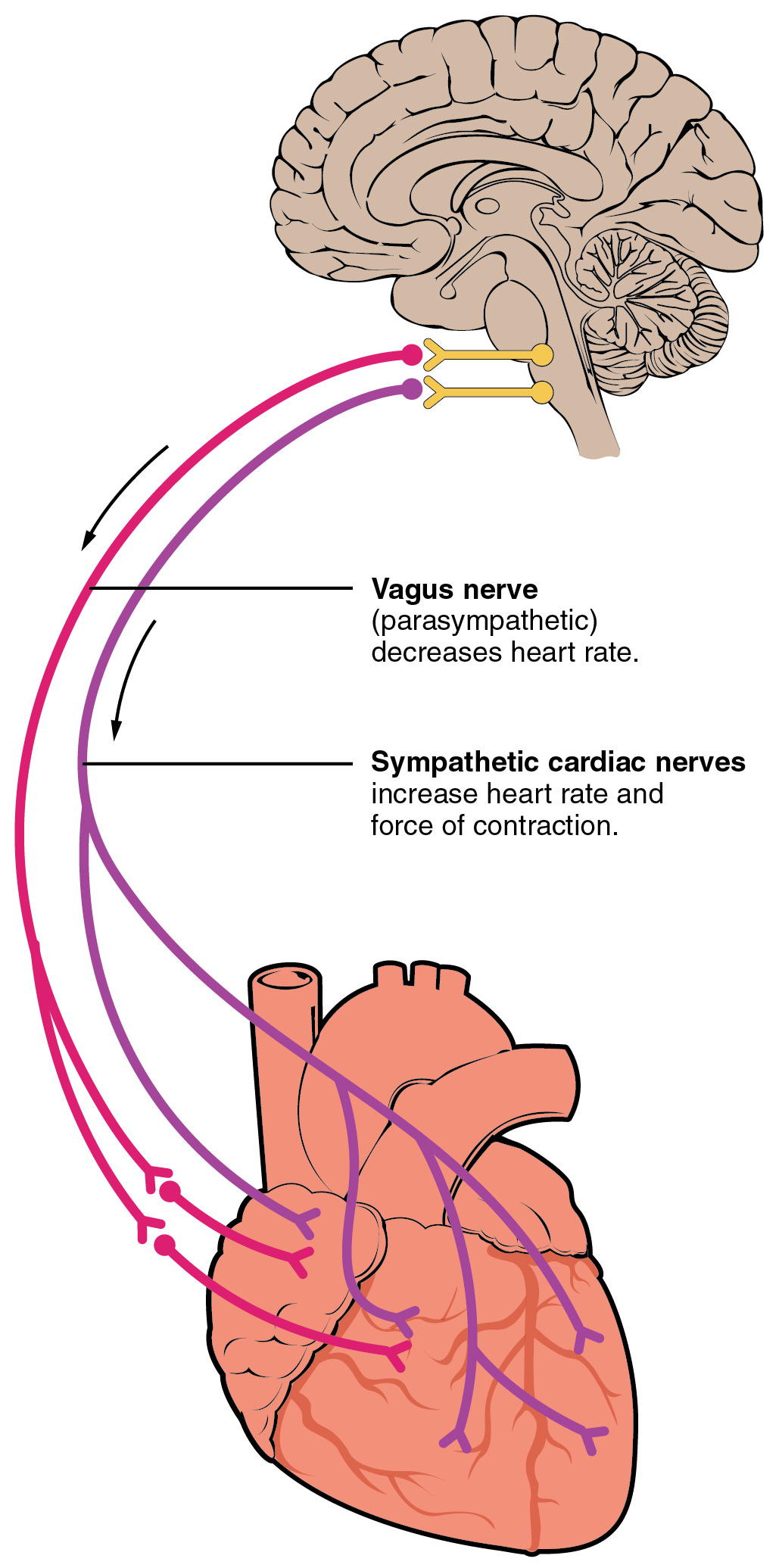
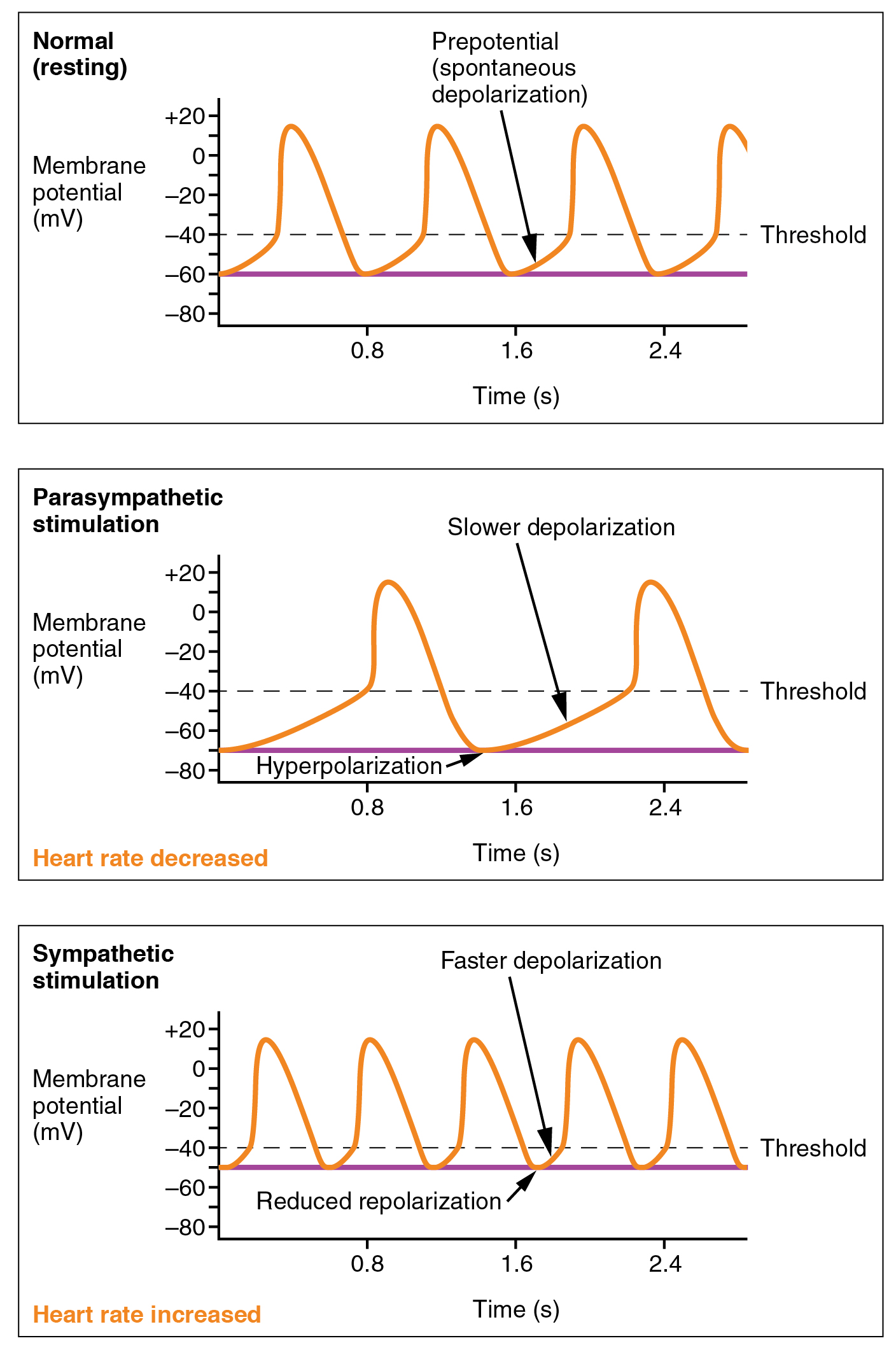
Parasympathetic stimulation originates from the cardioinhibitory region with impulses traveling via the vagus nerve (cranial nerve X). The vagus nerve sends branches to both the SA and AV nodes to decrease HR. Parasympathetic stimulation releases the neurotransmitter acetylcholine (ACh) at the neuromuscular junction. ACh slows HR by opening chemical- or ligand-gated potassium ion channels to slow the rate of spontaneous depolarization and increase the time before the next spontaneous depolarization occurs. Without any nervous stimulation, the SA node would establish a sinus rhythm of approximately 100 bpm. Since resting rates are considerably less than this, it becomes evident that parasympathetic stimulation normally slows HR. This is similar to an individual driving a car with one foot on the brake pedal. To speed up, one need merely remove one’s foot from the break and let the engine increase speed. In the case of the heart, decreasing parasympathetic stimulation decreases the release of ACh, which allows HR to increase up to approximately 100 bpm. Any increases beyond this rate would require sympathetic stimulation. Figure 19.4.3 illustrates the effects of parasympathetic and sympathetic stimulation on the normal sinus rhythm.
Input to the Cardiovascular Centers
The cardiovascular center receives input from the limbic system as well as a series of visceral receptors with impulses traveling through visceral sensory fibers within the vagus and sympathetic nerves via the cardiac plexus. Among these receptors are various proprioreceptors, baroreceptors, and chemoreceptors. Collectively, these inputs normally enable the cardiovascular centers to regulate heart function precisely, a process known as cardiac reflexes. Increased physical activity results in increased rates of firing by various proprioreceptors located in muscles, joint capsules, and tendons. Any such increase in physical activity would logically warrant increased blood flow. The cardiac centers monitor these increased rates of firing, and suppress parasympathetic stimulation and increase sympathetic stimulation as needed in order to increase blood flow.
Similarly, baroreceptors are stretch receptors located in the aortic sinus, carotid bodies, the venae cavae, and other locations, including pulmonary vessels and the rightatrium. Rates of firing from the baroreceptors represent blood pressure, level of physical activity, and the relative distribution of blood. The cardiac centers monitor baroreceptor firing to maintain cardiac homeostasis, a mechanism called the baroreceptor reflex. With increased pressure and stretch, the rate of baroreceptor firing increases, and the cardiac centers decrease sympathetic stimulation and increase parasympathetic stimulation. As pressure and stretch decrease, the rate of baroreceptor firing decreases, and the cardiac centers increase sympathetic stimulation and decrease parasympathetic stimulation.
There is a similar reflex, called the atrial reflex or Bainbridge reflex, associated with varying rates of blood flow to the atria. Increased venous return stretches the walls of the atria where specialized baroreceptors are located. However, as the atrial baroreceptors increase their rate of firing and as they stretch due to the increased blood pressure, the cardiac center responds by increasing sympathetic stimulation and inhibiting parasympathetic stimulation to increase HR.
Increased metabolic byproducts associated with increased activity, such as carbon dioxide, hydrogen ions, and lactic acid, plus falling oxygen levels, are detected by a suite of chemoreceptors innervated by the glossopharyngeal and vagus nerves. These chemoreceptors provide feedback to the cardiovascular centers about the need for increased or decreased blood flow, based on the relative levels of these substances.
The limbic system can also significantly impact HR related to emotional state. During periods of stress, it is not unusual to identify higher than normal HRs, often accompanied by a surge in the stress hormone cortisol. Individuals experiencing extreme anxiety may manifest panic attacks with symptoms that resemble those of heart attacks. These events are typically transient and treatable. Meditation techniques have been developed to ease anxiety and have been shown to lower HR effectively. Doing simple deep and slow breathing exercises with one’s eyes closed can also significantly reduce this anxiety and HR.
Other Factors Influencing Heart Rate and Force of Contraction
Using a combination of autorhythmicity and innervation, the cardiovascular centers are able to provide relatively precise control over HR. However, there are a number of other factors that have an impact on HR as well, including epinephrine, NE, and thyroid hormones; levels of various ions including calcium, potassium, and sodium; body temperature; hypoxia; and pH balance (Table 19.1 and Table 19.2). Many of these factors also influence contractility which refers to the force of contraction of the heart muscle. After reading this section, the importance of maintaining homeostasis should become even more apparent.
| Major Factors Increasing Heart Rate and Force of Contraction (Table 19.1) | |
|---|---|
| Factor | Effect |
| Cardioaccelerator nerves | Release of norepinephrine |
| Proprioreceptors | Increased rates of firing during exercise |
| Chemoreceptors | Decreased levels of O2; increased levels of H+, CO2, and lactic acid |
| Baroreceptors | Decreased rates of firing, indicating falling blood volume/pressure |
| Limbic system | Anticipation of physical exercise or strong emotions |
| Catecholamines | Increased epinephrine and norepinephrine |
| Thyroid hormones | Increased T3 and T4 |
| Calcium | Increased Ca2+ |
| Potassium | Decreased K+ |
| Sodium | Decreased Na+ |
| Body temperature | Increased body temperature |
| Nicotine and caffeine | Stimulants, increasing heart rate |
| Factors Decreasing Heart Rate and Force of Contraction (Table 19.2) | |
|---|---|
| Factor | Effect |
| Cardioinhibitor nerves (vagus) | Release of acetylcholine |
| Proprioreceptors | Decreased rates of firing following exercise |
| Chemoreceptors | Increased levels of O2; decreased levels of H+ and CO2 |
| Baroreceptors | Increased rates of firing, indicating higher blood volume/pressure |
| Limbic system | Anticipation of relaxation |
| Catecholamines | Decreased epinephrine and norepinephrine |
| Thyroid hormones | Decreased T3 and T4 |
| Calcium | Decreased Ca2+ |
| Potassium | Increased K+ |
| Sodium | Increased Na+ |
| Body temperature | Decrease in body temperature |
Epinephrine and Norepinephrine
The catecholamines, epinephrine and NE, secreted by the adrenal medulla form one component of the extended fight-or-flight mechanism. The other component is sympathetic stimulation. Epinephrine and NE have similar effects: binding to the beta-1 receptors, and opening sodium and calcium ion chemical- or ligand-gated channels. The rate of depolarization is increased by this additional influx of positively charged ions, so the threshold is reached more quickly increasing heart rate. However, massive releases of these hormones coupled with sympathetic stimulation may actually lead to arrhythmias. There is no parasympathetic stimulation to the adrenal medulla.
Thyroid Hormones
In general, increased levels of thyroid hormone, or thyroxin, increases HR and contractility. The impact of thyroid hormone is typically of a much longer duration than that of the catecholamines. The physiologically active form of thyroid hormone, T3 or triiodothyronine, has been shown to directly enter cardiomyocytes and alter activity at the level of the genome. It also impacts the beta adrenergic response similar to epinephrine and NE described above. Excessive levels of thyroxin may trigger tachycardia.
Calcium
Calcium ion levels have great impacts upon both HR and contractility; as the levels of calcium ions increase, so do HR and contractility. High levels of calcium ions (hypercalcemia) may be implicated in a short QT interval and a widened T wave in the ECG. The QT interval represents the time from the start of depolarization to repolarization of the ventricles, and includes the period of ventricular systole. As in skeletal muscle, increased calcium increases the force of contraction. Extremely high levels of calcium may induce cardiac arrest. Drugs known as calcium channel blockers slow HR by binding to these channels and blocking or slowing the inward movement of calcium ions.
Caffeine and Nicotine
Caffeine and nicotine are not found naturally within the body. Both of these drugs have an excitatory effect on membranes of neurons in general and have a stimulatory effect on the cardiac centers specifically, causing an increase in HR. Caffeine works by increasing the rates of depolarization at the SA node, whereas nicotine stimulates the activity of the sympathetic neurons that deliver impulses to the heart.
Although it is the world’s most widely consumed psychoactive drug, caffeine is legal and not regulated. While precise quantities have not been established, “normal” consumption is not considered harmful to most people, although it may cause disruptions to sleep and acts as a diuretic. Its consumption by pregnant women is cautioned against, although no evidence of negative effects has been confirmed. Tolerance and even physical and mental addiction to the drug result in individuals who routinely consume the substance.
Nicotine, too, is a stimulant and produces addiction. While legal, concerns about nicotine’s safety and documented links to respiratory and cardiac disease have resulted in warning labels on cigarette packages.
Sodium and Potassium
HR can be slowed when a person experiences altered sodium and potassium levels, hypoxia, acidosis, alkalosis, and hypothermia (see Table 19.1).
The relationship between electrolytes and HR is complex, but maintaining electrolyte balance is critical to the normal wave of depolarization. Of the two ions, potassium has the greater clinical significance. Hypokalemia (low potassium levels) leads to arrhythmias, whereas hyperkalemia (high potassium levels) causes the heart to become weak and flaccid, and ultimately to fail. Initially, both hyponatremia (low sodium levels) and hypernatremia (high sodium levels) may lead to tachycardia. Severely high hypernatremia may lead to fibrillation, which may cause CO to cease. Severe hyponatremia leads to both bradycardia and other arrhythmias.
Other Factors
Heart muscle relies exclusively on aerobic metabolism for energy. Hypoxia (an insufficient supply of oxygen) leads to decreasing HRs, since metabolic reactions fueling heart contraction are restricted.
Acidosis is a condition in which excess hydrogen ions are present, and the patient’s blood expresses a low pH value. Alkalosis is a condition in which there are too few hydrogen ions, and the patient’s blood has an elevated pH. Normal blood pH falls in the range of 7.35–7.45, so a number lower than this range represents acidosis and a higher number represents alkalosis. Recall that enzymes are the regulators or catalysts of virtually all biochemical reactions; they are sensitive to pH and will change shape slightly with values outside their normal range. These variations in pH and accompanying slight physical changes to the active site on the enzyme decrease the rate of formation of the enzyme-substrate complex, subsequently decreasing the rate of many enzymatic reactions, which can have complex effects on HR. Severe changes in pH will lead to denaturation of the enzyme.
The last variable is body temperature. Elevated body temperature is called hyperthermia, and suppressed body temperature is called hypothermia. Slight hyperthermia results in increasing HR and strength of contraction. Hypothermia slows the rate and strength of heart contractions. This distinct slowing of the heart is one component of the larger diving reflex that diverts blood to essential organs while submerged in cold water. If sufficiently chilled, the heart will stop beating, a technique that may be employed during open heart surgery. In this case, the patient’s blood is normally diverted to an artificial heart-lung machine to maintain the body’s blood supply and gas exchange until the surgery is complete, and sinus rhythm can be restored. Excessive hyperthermia and hypothermia will both result in death, as enzymes drive the body systems to cease normal function, beginning with the central nervous system.
Stroke Volume
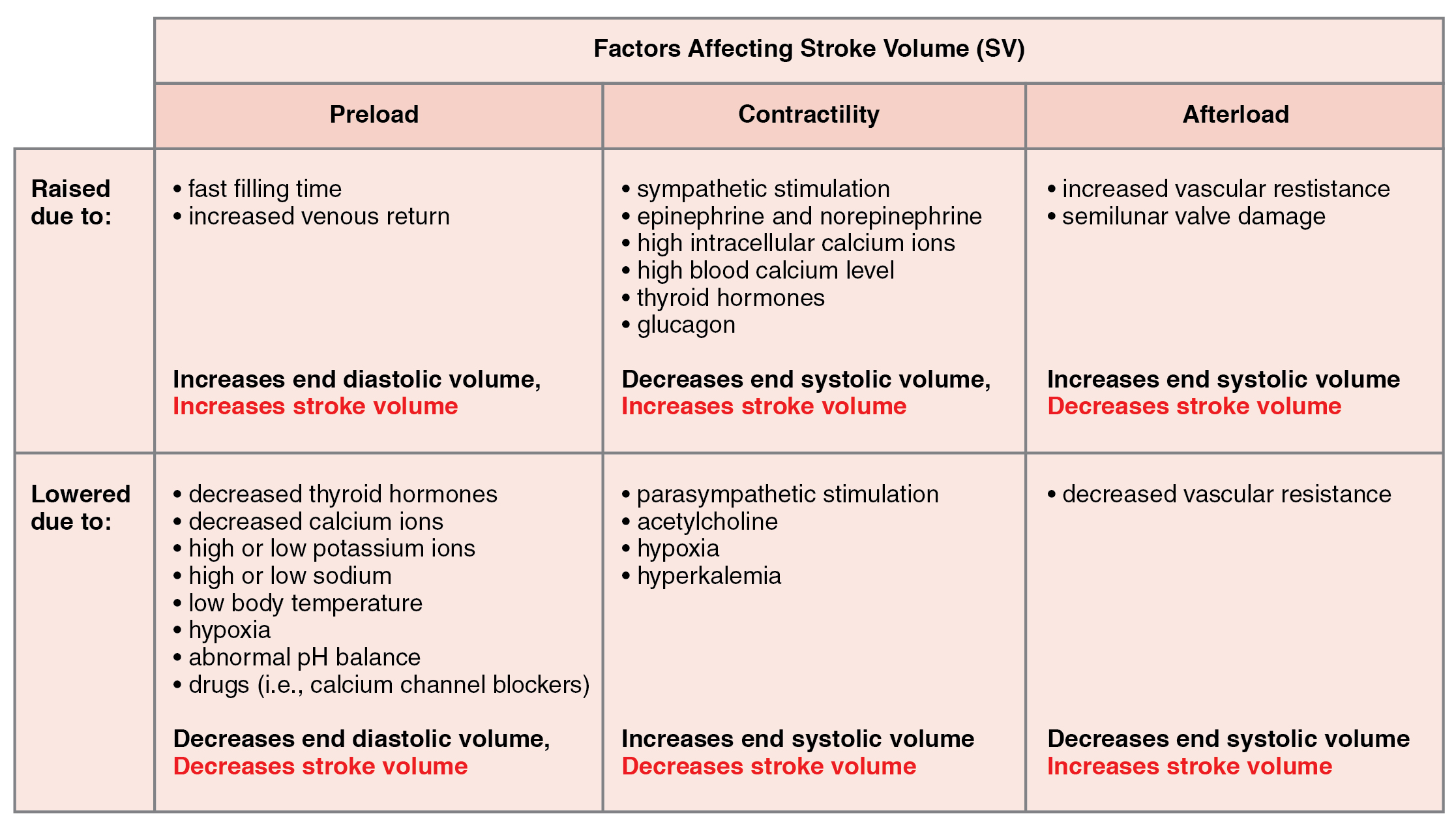
Many of the same factors that regulate HR also impact cardiac function by altering SV. While a number of variables are involved, SV is ultimately dependent upon the difference between EDV and ESV. The three primary factors to consider are preload, or the stretch on the ventricles prior to contraction; the contractility, or the force or strength of the contraction itself; and afterload, the force the ventricles must generate to pump blood against the resistance in the vessels. These factors are summarized in Table 19.1 and Table 19.2.
Preload
Preload is the degree to which cardiac muscle cells are stretched from filling of the ventricles prior to contraction. Therefore preload is another way of expressing EDV. With increasing ventricular filling, both EDV or preload increase, and the cardiac muscle itself is stretched to a greater degree. At rest, there is little stretch of the ventricular muscle, and the sarcomeres remain short. With increased ventricular filling, the ventricular muscle is increasingly stretched and the sarcomere length increases. As the sarcomeres reach their optimal lengths, they will contract more powerfully, because more of the myosin heads can bind to the actin on the thin filaments, forming cross bridges and increasing the strength of contraction and SV. If this process were to continue and the sarcomeres stretched beyond their optimal lengths, the force of contraction would decrease. However, due to the physical constraints of the location of the heart, this excessive stretch is not a concern.
One of the primary factors to consider is filling time, or the duration of ventricular diastole during which filling occurs. The more rapidly the heart contracts, the shorter the filling time becomes, and the lower the EDV and preload are. This effect can be partially overcome by increasing the second variable, contractility, which raises the SV, but over time, the heart is unable to compensate for decreased filling time, and preload also decreases.
The relationship between ventricular stretch and contraction has been stated in the well-known Frank-Starling mechanism or simply Starling’s Law of the Heart. This principle states that, within physiological limits, the force of heart contraction is directly proportional to the initial length of the muscle fiber. This means that the greater the stretch of the ventricular muscle (within limits), the more powerful the contraction is, which in turn increases SV. Therefore, by increasing preload, you increase the second variable, contractility.
Otto Frank (1865–1944) was a German physiologist; among his many published works are detailed studies of this important heart relationship. Ernest Starling (1866–1927) was an important English physiologist who also studied the heart. Although they worked largely independently, their combined efforts and similar conclusions have been recognized in the name “Frank-Starling mechanism.”
Any sympathetic stimulation to the venous system will increase venous return to the heart, which contributes to ventricular filling, and EDV and preload. While much of the ventricular filling occurs while both atria and ventricles are in diastole, the contraction of the atria, the atrial kick (refer to Figure 3, section 19.3 ), plays a crucial role by providing the last 20–30 percent of ventricular filling.
Contractility
It is virtually impossible to consider preload or ESV without including the concept of contractility. Indeed, the two parameters are intimately linked. Contractility refers to the force of the contraction of the heart muscle, which controls SV, and is the primary parameter for impacting ESV. The more forceful the contraction is, the greater the SV and smaller the ESV are. Less forceful contractions result in smaller SVs and larger ESVs. Factors that increase contractility are described as positive inotropic factors, and those that decrease contractility are described as negative inotropic factors (ino- = “fiber;” -tropic = “turning toward”).
Not surprisingly, sympathetic stimulation is a positive inotrope, whereas parasympathetic stimulation is a negative inotrope. Sympathetic stimulation triggers the release of NE at the neuromuscular junction from the cardiac nerves and also stimulates the adrenal cortex to secrete epinephrine and NE. In addition to their stimulatory effects on HR, as positive chronotropic factors, they also bind to both alpha and beta receptors on the cardiac muscle cell membrane to increase metabolic rate and the force of contraction. This combination of actions has the net effect of increasing SV and leaving a smaller residual ESV in the ventricles. In comparison, parasympathetic stimulation releases ACh at the neuromuscular junction from the vagus nerve. The membrane hyperpolarizes and decreases contraction to decrease the strength of contraction and SV, and to raise ESV. Since parasympathetic fibers are more widespread in the atria than in the ventricles, the primary site of action is in the upper chambers. Parasympathetic stimulation in the atria decreases the atrial kick and reduces EDV, which decreases ventricular stretch and preload, thereby further limiting the force of ventricular contraction. Stronger parasympathetic stimulation also directly decreases the force of contraction of the ventricles.
Several synthetic drugs, including dopamine and isoproterenol, have been developed that mimic the effects of epinephrine and NE by stimulating the influx of calcium ions from the extracellular fluid. Higher concentrations of intracellular calcium ions increase the strength of contraction. Excess calcium (hypercalcemia) also acts as a positive inotropic agent. The drug digitalis is a negative chronotropic factor because it lowers HR and it increases the strength of the contraction, acting as a positive inotropic agent by blocking the sequestering of calcium ions into the sarcoplasmic reticulum. This leads to higher intracellular calcium levels and greater strength of contraction. In addition to the catecholamines from the adrenal medulla, other hormones also demonstrate positive inotropic effects. These include thyroid hormones and glucagon from the pancreas.
Negative inotropic agents include hypoxia, acidosis, hyperkalemia, and a variety of synthetic drugs. These include numerous beta blockers and calcium channel blockers. Early beta blocker drugs include propranolol and pronethalol, and are credited with revolutionizing treatment of cardiac patients experiencing angina pectoris. There is also a large class of dihydropyridine, phenylalkylamine, and benzothiazepine calcium channel blockers that may be administered decreasing the strength of contraction and SV.
Afterload
Afterload refers to the tension or force that the ventricles must develop to pump blood effectively against the resistance in the vascular system. Any condition that increases resistance such as vasoconstriction or the disease atherosclerosis requires a greater afterload to force open the semilunar valves and pump the blood. Damage to the valves, such as stenosis, which makes them harder to open will also increase afterload. Any decrease in resistance as with vasodilation, decreases the afterload. Figure 19.4.4 summarizes the major factors influencing SV, Figure 19.4.5 summarizes the major factors influencing CO, and Table 19.3 and Table 19.4 summarize cardiac responses to increased and decreased blood flow and pressure in order to restore homeostasis.
| Summary of Cardiac Response to Decreasing Blood Flow and Pressure Due to Decreasing Cardiac Output (Table 19.3) | ||
|---|---|---|
| Baroreceptors (aorta, carotid arteries, venae cavae, and atria) | Chemoreceptors (both central nervous system and in proximity to baroreceptors) | |
| Sensitive to | Decreasing stretch | Decreasing O2 and increasing CO2, H+, and lactic acid |
| Action | Parasympathetic stimulation suppressed | Sympathetic stimulation increased |
| Effect on Heart | Increasing heart rate and increasing stroke volume | Increasing heart rate and increasing stroke volume |
| Overall effect | Increasing blood flow and pressure due to increasing cardiac output; homeostasis restored | Increasing blood flow and pressure due to increasing cardiac output; homeostasis restored |
| Summary of Cardiac Response to Increasing Blood Flow and Pressure Due to Increasing Cardiac Output (Table 19.4) | ||
|---|---|---|
| Baroreceptors (aorta, carotid arteries, venae cavae, and atria) | Chemoreceptors (both central nervous system and in proximity to baroreceptors) | |
| Sensitive to | Increasing stretch | Increasing O2 and decreasing CO2, H+, and lactic acid |
| Action | Parasympathetic stimulation increased | Sympathetic stimulation suppressed |
| Effect on heart | Decreasing heart rate and decreasing stroke volume | Decreasing heart rate and decreasing stroke volume |
| Overall effect | Decreasing blood flow and pressure due to decreasing cardiac output; homeostasis restored | Decreasing blood flow and pressure due to decreasing cardiac output; homeostasis restored |
Chapter Review
Many factors affect HR and SV, and together, they contribute to cardiac function. HR is largely determined and regulated by autonomic stimulation and hormones. There are several feedback loops that contribute to maintaining homeostasis dependent upon activity levels, such as the atrial reflex, which is determined by venous return.
SV is regulated by autonomic innervation and hormones, but also by filling time and venous return. Venous return is determined by activity of the skeletal muscles, blood volume, and changes in peripheral circulation. Venous return determines preload and the atrial reflex. Filling time directly related to HR also determines preload. Preload then impacts both EDV and ESV. Autonomic innervation and hormones largely regulate contractility. Contractility impacts EDV as does afterload. CO is the product of HR multiplied by SV. SV is the difference between EDV and ESV.
Review Questions
Critical Thinking Questions
1. Why does increasing EDV increase contractility?
2. Why is afterload important to cardiac function?
Glossary
- afterload
- force the ventricles must develop to effectively pump blood against the resistance in the vessels
- autonomic tone
- contractile state during resting cardiac activity produced by mild sympathetic and parasympathetic stimulation
- atrial or Bainbridge reflex
- autonomic reflex that responds to stretch receptors in the atria that send impulses to the cardioaccelerator area to increase HR when venous flow into the atria increases
- baroreceptor reflex
- autonomic reflex in which the cardiac centers monitor signals from the baroreceptor stretch receptors and regulate heart function based on blood flow
- cardiac output (CO)
- amount of blood pumped by each ventricle during one minute; equals HR multiplied by SV
- cardiac plexus
- paired complex network of nerve fibers near the base of the heart that receive sympathetic and parasympathetic stimulations to regulate HR
- cardiac reflexes
- series of autonomic reflexes that enable the cardiovascular centers to regulate heart function based upon sensory information from a variety of visceral sensors
- cardiac reserve
- difference between maximum and resting CO
- chronotropic factor
- a factor that affects heart rate
- ejection fraction
- portion of the blood that is pumped or ejected from the heart with each contraction; mathematically represented by SV divided by EDV
- filling time
- duration of ventricular diastole during which filling occurs
- Frank-Starling mechanism
- relationship between ventricular stretch and contraction in which the force of heart contraction is directly proportional to the initial length of the muscle fiber
- heart rate (HR)
- number of times the heart contracts (beats) per minute
- negative chronotropic factors
- factors that reduce heart rate
- negative inotropic factors
- factors that negatively impact or lower heart contractility
- positive chronotropic factors
- factors that increase heart rate
- positive inotropic factors
- factors that positively impact or increase heart contractility
- stroke volume (SV)
- amount of blood pumped by each ventricle per contraction; also, the difference between EDV and ESV
- target heart rate
- range in which both the heart and lungs receive the maximum benefit from an aerobic workout
Solutions
Answers for Critical Thinking Questions
- Increasing EDV increases the sarcomeres’ lengths within the cardiac muscle cells, allowing more cross bridge formation between the myosin and actin and providing for a more powerful contraction. This relationship is described in the Frank-Starling mechanism.
- Afterload represents the resistance within the arteries to the flow of blood ejected from the ventricles. If uncompensated, if afterload increases, flow will decrease. In order for the heart to maintain adequate flow to overcome increasing afterload, it must pump more forcefully. This is one of the negative consequences of high blood pressure or hypertension.
This work, Anatomy & Physiology, is adapted from Anatomy & Physiology by OpenStax, licensed under CC BY. This edition, with revised content and artwork, is licensed under CC BY-SA except where otherwise noted.
Images, from Anatomy & Physiology by OpenStax, are licensed under CC BY except where otherwise noted.
Access the original for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction.

