20.4 Parasitic Infections of the Circulatory and Lymphatic Systems
Learning Objectives
- Identify common parasites that cause infections of the circulatory and lymphatic systems
- Compare the major characteristics of specific parasitic diseases affecting the circulatory and lymphatic systems
Some protozoa and parasitic flukes are also capable of causing infections of the human circulatory system. Although these infections are rare in the US, they continue to cause widespread suffering in the developing world today. Fungal infections of the circulatory system are very rare. Therefore, they are not discussed in this chapter.
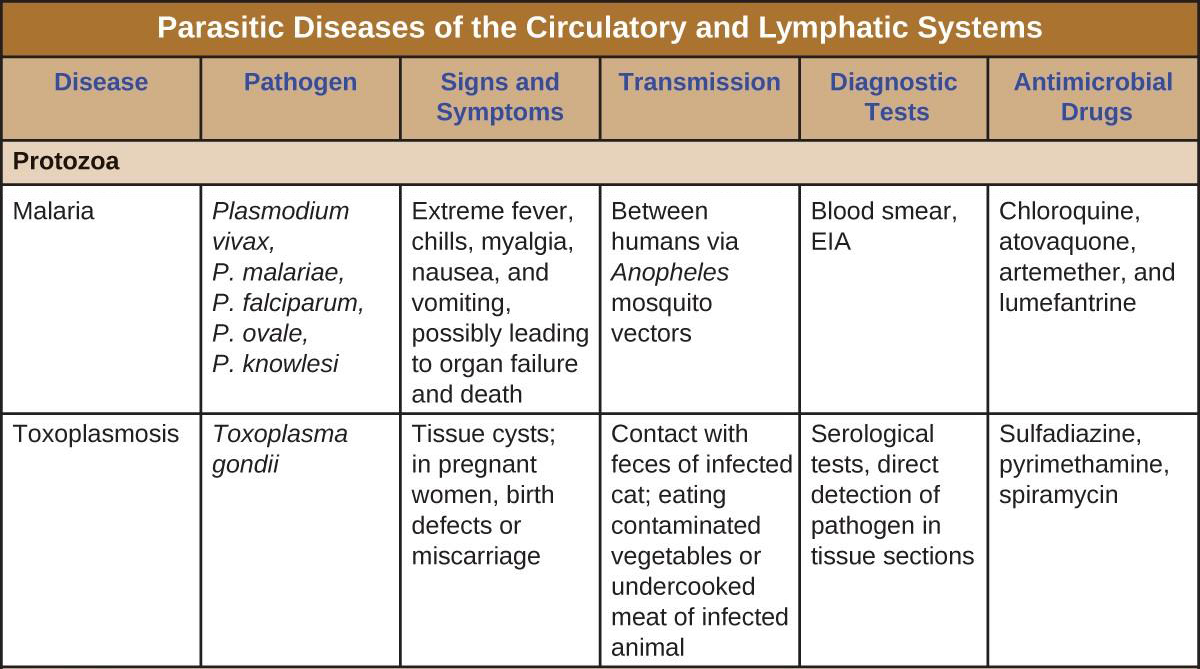
Malaria
Despite more than a century of intense research and clinical advancements, malaria remains one of the most important infectious diseases in the world today. Its widespread distribution places more than half of the world’s population in jeopardy. In 2015, the WHO estimated there were about 214 million cases of malaria worldwide, resulting in about 438,000 deaths; about 88% of cases and 91% of deaths occurred in Africa.[1] Although malaria is not currently a major threat in the US, the possibility of its reintroduction is a concern. Malaria is caused by several protozoan parasites in the genus Plasmodium: P. falciparum, P. knowlesi, P. malariae, P. ovale, and P. vivax. Plasmodium primarily infect red blood cells and are transmitted through the bite of Anopheles mosquitoes.
Currently, P. falciparum is the most common and most lethal cause of malaria, often called falciparum malaria. Falciparum malaria is widespread in highly populated regions of Africa and Asia, putting many people at risk for the most severe form of the disease.
The classic signs and symptoms of malaria are cycles of extreme fever and chills. The sudden, violent symptoms of malaria start with malaise, abrupt chills, and fever (39–41° C [102.2–105.8 °F]), rapid and faint pulse, polyuria, headache, myalgia, nausea, and vomiting. After 2 to 6 hours of these symptoms, the fever falls, and profuse sweating occurs for 2 to 3 hours, followed by extreme fatigue. These symptoms are a result of Plasmodium emerging from red blood cells synchronously, leading to simultaneous rupture of a large number of red blood cells, resulting in damage to the spleen, liver, lymph nodes, and bone marrow. The organ damage resulting from hemolysis causes patients to develop sludge blood (i.e., blood in which the red blood cells agglutinate into clumps) that can lead to lack of oxygen, necrosis of blood vessels, organ failure, and death.
In established infections, malarial cycles of fever and chills typically occur every 2 days in the disease described as tertian malaria, which is caused by P. vivax and P. ovale. The cycles occur every 3 days in the disease described as quartan malaria, which is caused by P. malariae. These intervals may vary among cases.
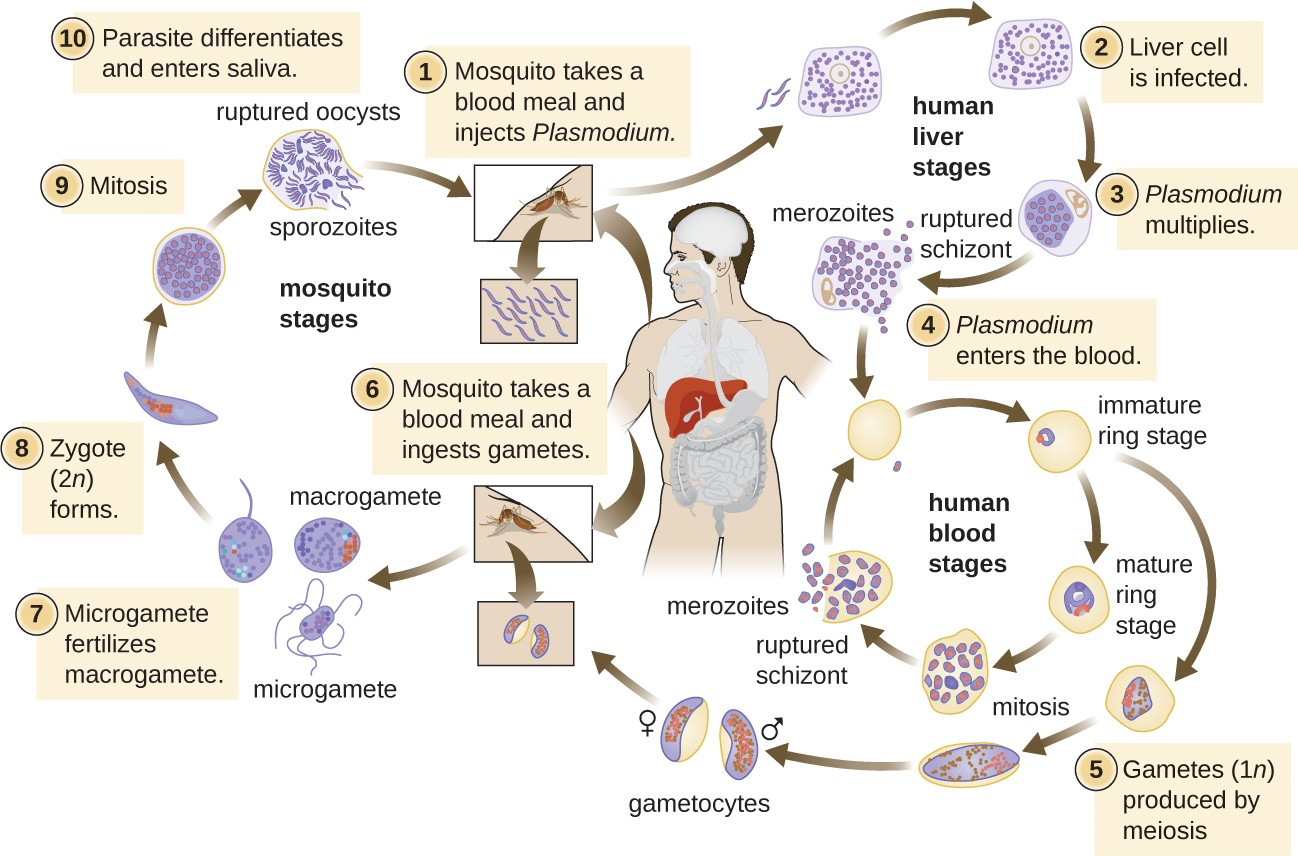
Plasmodium has a complex life cycle that includes several developmental stages alternately produced in mosquitoes and humans (Figure 20.18). When an infected mosquito takes a blood meal, sporozoites in the mosquito salivary gland are injected into the host’s blood. These parasites circulate to the liver, where they develop into schizonts. The schizonts then undergo schizogony, resulting in the release of many merozoites at once. The merozoites move to the bloodstream and infect red blood cells. Inside red blood cells, merozoites develop into trophozoites that produce more merozoites. The synchronous release of merozoites from red blood cells in the evening leads to the symptoms of malaria.
In addition, some trophozoites alternatively develop into male and female gametocytes. The gametocytes are taken up when the mosquito takes a blood meal from an infected individual. Sexual sporogony occurs in the gut of the mosquito. The gametocytes fuse to form zygotes in the insect gut. The zygotes become motile and elongate into an ookinete. This form penetrates the midgut wall and develops into an oocyst. Finally, the oocyst releases new sporozoites that migrate to the mosquito salivary glands to complete the life cycle.
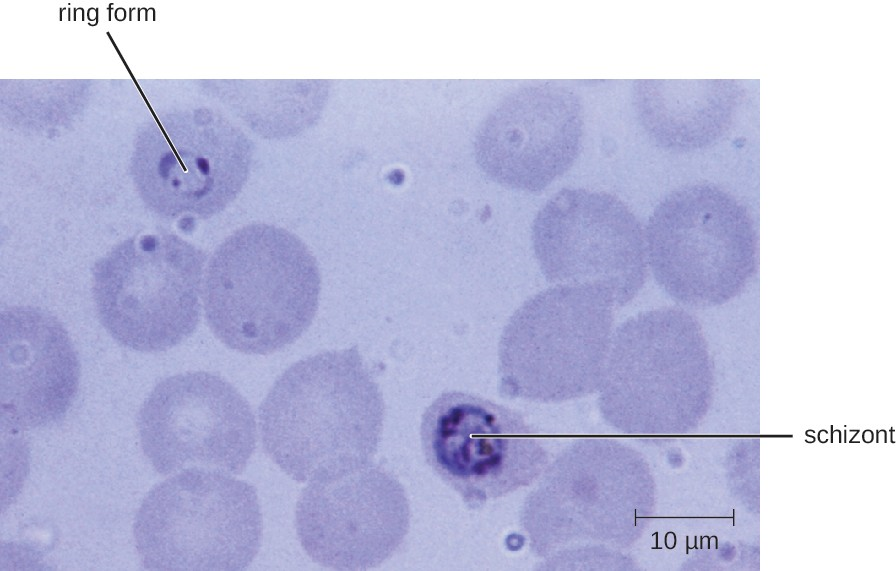
Diagnosis of malaria is by microscopic observation of developmental forms of Plasmodium in blood smears and rapid EIA assays that detect Plasmodium antigens or enzymes (Figure 20.19). Drugs such as chloroquine, atovaquone , artemether, and lumefantrine may be prescribed for both acute and prophylactic therapy, although some Plasmodium spp. have shown resistance to antimalarial drugs. Use of insecticides and insecticide-treated bed nets can limit the spread of malaria. Despite efforts to develop a vaccine for malaria, none is currently available.
Link to Learning
Visit this site (https://openstax.org/l/22plasmodium) to learn how the parasite Plasmodium infects red blood cells.
The Nothing But Nets campaign, an initiative of the United Nations Foundation, has partnered with the Bill and Melinda Gates Foundation to make mosquito bed nets available in developing countries in Africa.
Visit their website (https://openstax.org/l/22mosquitonet) to learn more about their efforts to prevent malaria.
![]()
- Why is malaria one of the most important infectious diseases?
Toxoplasmosis
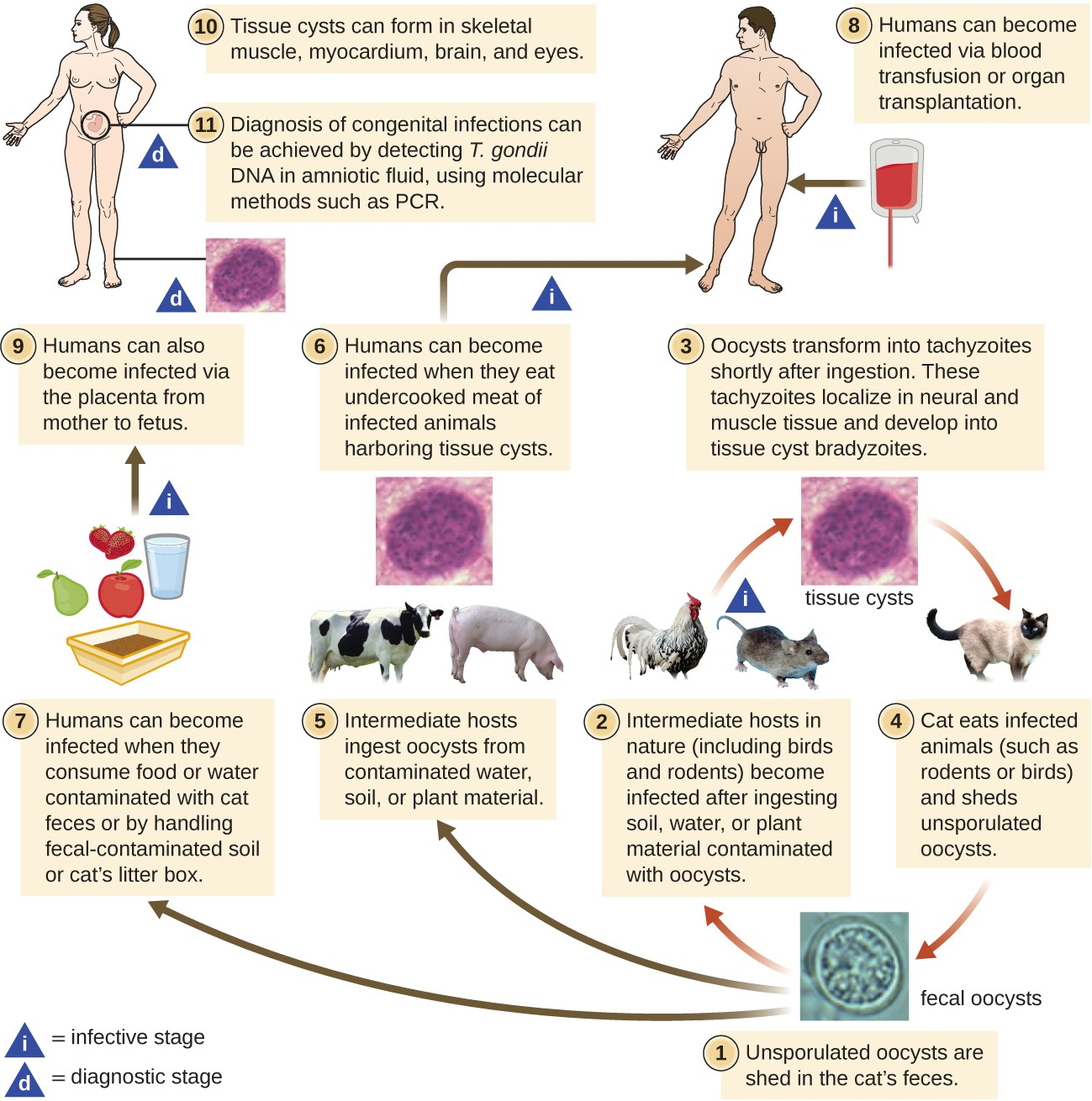
The disease toxoplasmosis is caused by the protozoan Toxoplasma gondii. T. gondii is found in a wide variety of birds and mammals,[2] and human infections are common. The Centers for Disease Control and Prevention (CDC) estimates that 22.5% of the population 12 years and older has been infected with T. gondii; but immunocompetent individuals are typically asymptomatic, however.[3] Domestic cats are the only known definitive hosts for the sexual stages of T. gondii and, thus, are the main reservoirs of infection. Infected cats shed T. gondii oocysts in their feces, and these oocysts typically spread to humans through contact with fecal matter on cats’ bodies, in litter boxes, or in garden beds where outdoor cats defecate.
T. gondii has a complex life cycle that involves multiple hosts. The T. gondii life cycle begins when unsporulated oocysts are shed in the cat’s feces. These oocysts take 1–5 days to sporulate in the environment and become infective. Intermediate hosts in nature include birds and rodents, which become infected after ingesting soil, water, or plant material contaminated with the infective oocysts. Once ingested, the oocysts transform into tachyzoites that localize in the bird or rodent neural and muscle tissue, where they develop into tissue cysts. Cats may become infected after consuming birds and rodents harboring tissue cysts. Cats and other animals may also become infected directly by ingestion of sporulated oocysts in the environment. Interestingly, Toxoplasma infection appears to be able to modify the host’s behavior. Mice infected by Toxoplasma lose their fear of cat pheromones. As a result, they become easier prey for cats, facilitating the transmission of the parasite to the cat definitive host[4] (Figure 20.20).
Toxoplasma infections in humans are extremely common, but most infected people are asymptomatic or have subclinical symptoms. Some studies suggest that the parasite may be able to influence the personality and psychomotor performance of infected humans, similar to the way it modifies behavior in other mammals.[5] When symptoms do occur, they tend to be mild and similar to those of mononucleosis. However, asymptomatic toxoplasmosis can become problematic in certain situations. Cysts can lodge in a variety of human tissues and lie dormant for years. Reactivation of these quiescent infections can occur in immunocompromised patients following transplantation, cancer therapy, or the development of an immune disorder such as AIDS. In patients with AIDS who have toxoplasmosis, the immune system cannot combat the growth of T. gondii in body tissues; as a result, these cysts can cause encephalitis, retinitis, pneumonitis, cognitive disorders, and seizures that can eventually be fatal.
Toxoplasmosis can also pose a risk during pregnancy because tachyzoites can cross the placenta and cause serious infections in the developing fetus. The extent of fetal damage resulting from toxoplasmosis depends on the severity of maternal disease, the damage to the placenta, the gestational age of the fetus when infected, and the virulence of the organism. Congenital toxoplasmosis often leads to fetal loss or premature birth and can result in damage to the central nervous system, manifesting as mental retardation, deafness, or blindness. Consequently, pregnant women are advised by the CDC to take particular care in preparing meat, gardening, and caring for pet cats.[6] Diagnosis of toxoplasmosis infection during pregnancy is usually achieved by serology including TORCH testing (the “T” in TORCH stands for toxoplasmosis). Diagnosis of congenital infections can also be achieved by detecting T. gondii DNA in amniotic fluid, using molecular methods such as PCR.
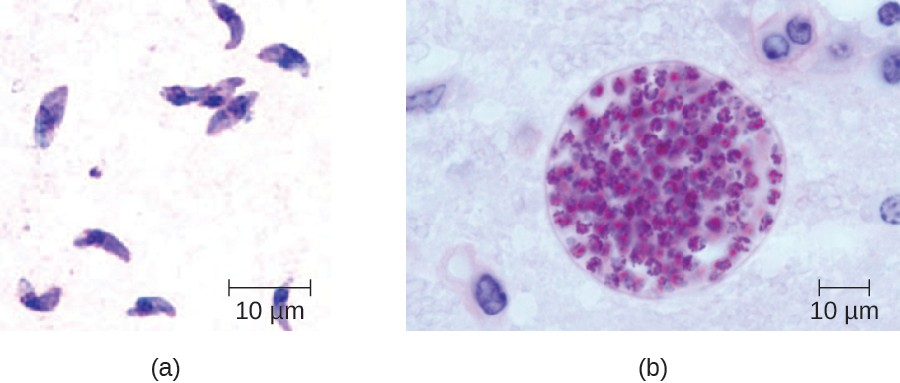
In adults, diagnosis of toxoplasmosis can include observation of tissue cysts in tissue specimens. Tissue cysts may be observed in Giemsa- or Wright-stained biopsy specimens, and CT, magnetic resonance imaging, and lumbar puncture can also be used to confirm infection (Figure 20.21).
Preventing infection is the best first-line defense against toxoplasmosis. Preventive measures include washing hands thoroughly after handling raw meat, soil, or cat litter, and avoiding consumption of vegetables possibly contaminated with cat feces. All meat should be cooked to an internal temperature of 73.9–76.7 °C (165–170 °F).
Most immunocompetent patients do not require clinical intervention for Toxoplasma infections. However, neonates, pregnant women, and immunocompromised patients can be treated with pyrimethamine and sulfadiazine—except during the first trimester of pregnancy, because these drugs can cause birth defects. Spiramycin has been used safely to reduce transmission in pregnant women with primary infection during the first trimester because it does not cross the placenta.
![]()
- How does T. gondii infect humans?
- World Health Organization. “World Malaria Report 2015: Summary.” 2015. http://www.who.int/malaria/publications/world-malaria- report-2015/report/en/. Accessed July 28, 2016. ↵
- A.M. Tenter et al.. “Toxoplasma gondii: From Animals to Humans.” International Journal for Parasitology 30 no. 12-13 (2000):1217–1258. ↵
- Centers for Disease Control and Prevention. “Parasites - Toxoplasmosis (Toxoplasma Infection). Epidemiology & Risk Factors.” 2015 http://www.cdc.gov/parasites/toxoplasmosis/epi.html. Accessed July 28, 2016. ↵
- J. Flegr. “Effects of Toxoplasma on Human Behavior.” Schizophrenia Bulletin 33, no. 3 (2007):757–760. ↵
- Ibid ↵
- Centers for Disease Control and Prevention. “Parasites - Toxoplasmosis (Toxoplasma infection). Toxoplasmosis Frequently Asked Questions (FAQs).” 2013. http://www.cdc.gov/parasites/toxoplasmosis/gen_info/faqs.html. Accessed July 28, 2016. ↵

