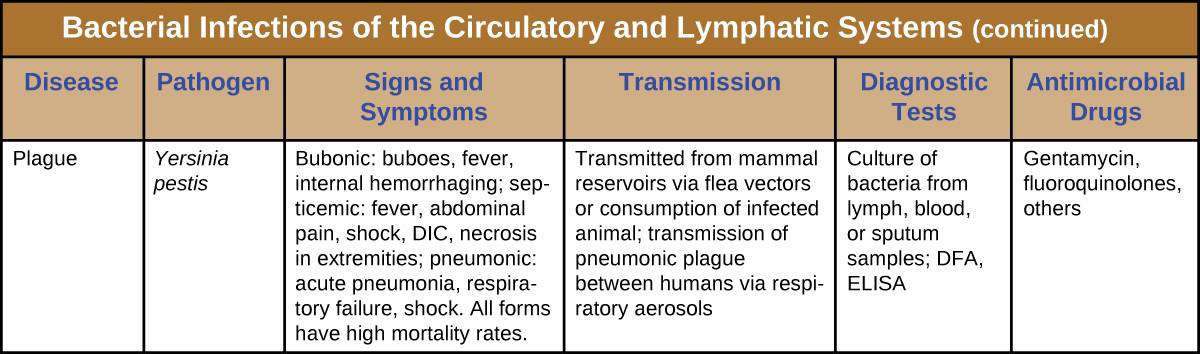
20.2 Bacterial Infections of the Circulatory and Lymphatic Systems
Learning Objectives
- Identify and compare bacteria that most commonly cause infections of the circulatory and lymphatic systems
- Compare the major characteristics of specific bacterial diseases affecting the circulatory and lymphatic systems
Bacteria can enter the circulatory and lymphatic systems through acute infections or breaches of the skin barrier or mucosa. Breaches may occur through fairly common occurrences, such as insect bites or small wounds. Even the act of tooth brushing, which can cause small ruptures in the gums, may introduce bacteria into the circulatory system. Inmost cases, the bacteremia that results from such common exposures is transient and remains below the threshold of detection. In severe cases, bacteremia can lead to septicemia with dangerous complications such as toxemia, sepsis, and septic shock. In these situations, it is often the immune response to the infection that results in the clinical signs and symptoms rather than the microbes themselves.
Plague
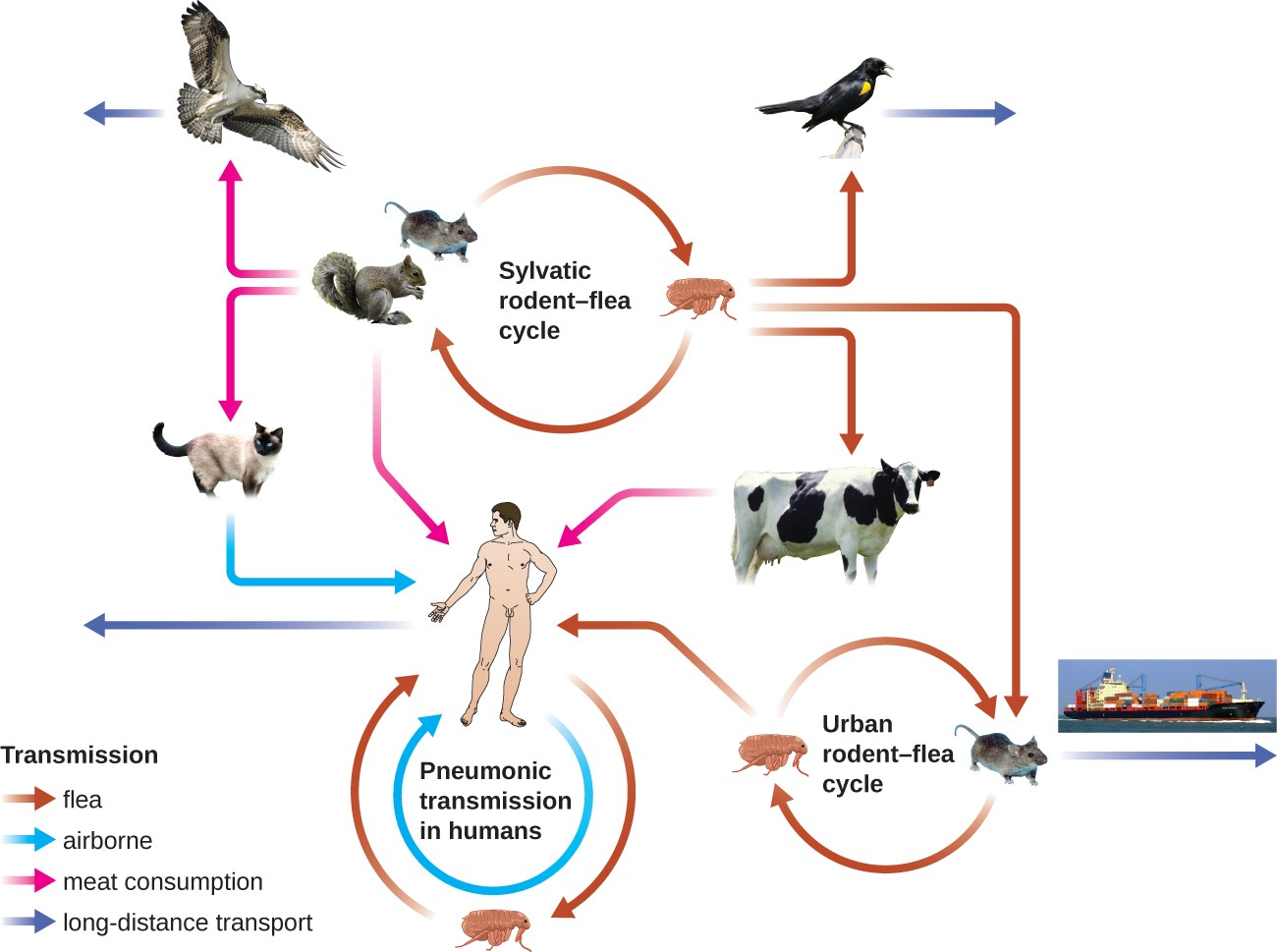
The gram-negative bacillus Yersinia pestis causes the zoonotic infection plague. This bacterium causes acute febrile disease in animals, usually rodents or other small mammals, and humans. The disease is associated with a high mortality rate if left untreated. Historically, Y. pestis has been responsible for several devastating pandemics, resulting in millions of deaths (see Micro Connections: The History of the Plague). There are three forms of plague: bubonic plague (the most common form, accounting for about 80% of cases), pneumonic plague, and septicemic plague. These forms are differentiated by the mode of transmission and the initial site of infection. Figure 20.6 illustrates these various modes of transmission and infection between animals and humans.
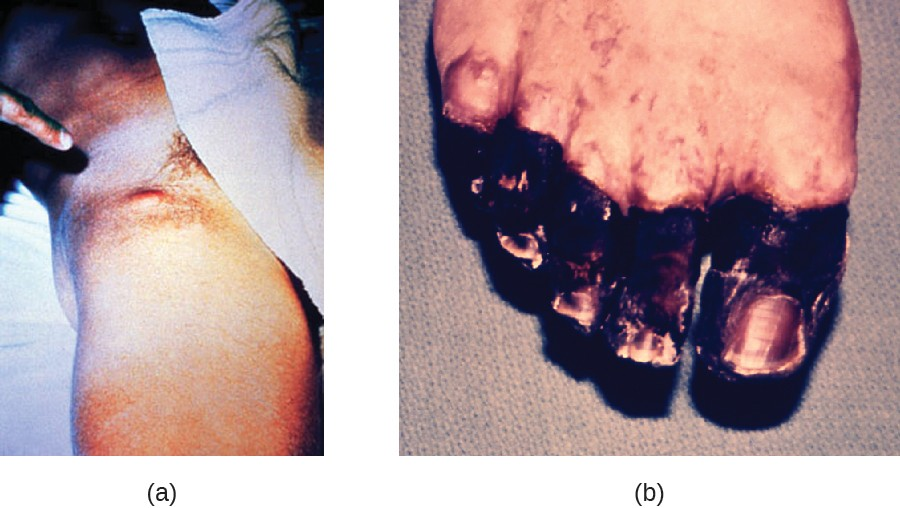
In bubonic plague, Y. pestis is transferred by the bite of infected fleas. Since most flea bites occur on the legs and ankles, Y. pestis is often introduced into the tissues and blood circulation in the lower extremities. After a 2- to 6-day incubation period, patients experience an abrupt onset fever (39.5–41 °C [103.1–105.8 °F]), headache, hypotension, and chills. The pathogen localizes in lymph nodes, where it causes inflammation, swelling, and hemorrhaging that results in purple buboes (Figure 20.7). Buboes often form in lymph nodes of the groin first because these are the nodes associated with the lower limbs; eventually, through circulation in the blood and lymph, lymph nodes throughout the body become infected and form buboes. The average mortality rate for bubonic plague is about 55% if untreated and about 10% with antibiotic treatment.
Septicemic plague occurs when Y. pestis is directly introduced into the bloodstream through a cut or wound and circulates through the body. The incubation period for septicemic plague is 1 to 3 days, after which patients develop fever, chills, extreme weakness, abdominal pain, and shock. Disseminated intravascular coagulation (DIC) can also occur, resulting in the formation of thrombi that obstruct blood vessels and promote ischemia and necrosis in surrounding tissues (Figure 20.7). Necrosis occurs most commonly in extremities such as fingers and toes, which become blackened. Septicemic plague can quickly lead to death, with a mortality rate near 100% when it is untreated. Even with antibiotic treatment, the mortality rate is about 50%.
Pneumonic plague occurs when Y. pestis causes an infection of the lungs. This can occur through inhalation of aerosolized droplets from an infected individual or when the infection spreads to the lungs from elsewhere in the body in patients with bubonic or septicemic plague. After an incubation period of 1 to 3 days, signs and symptoms include fever, headache, weakness, and a rapidly developing pneumonia with shortness of breath, chest pain, and cough producing bloody or watery mucus. The pneumonia may result in rapid respiratory failure and shock. Pneumonic plague is the only form of plague that can be spread from person to person by infectious aerosol droplet. If untreated, the mortality rate is near 100%; with antibiotic treatment, the mortality rate is about 50%.
The high mortality rate for the plague is, in part, a consequence of it being unusually well equipped with virulence factors. To date, there are at least 15 different major virulence factors that have been identified from Y. pestis and, of these, eight are involved with adherence to host cells. In addition, a component of the Y. pestis capsule is a virulence factor that allows the bacterium to avoid phagocytosis. Successful use of virulence factors allows the bacilli to disseminate from the area of the bite to regional lymph nodes and eventually the entire blood and lymphatic systems.
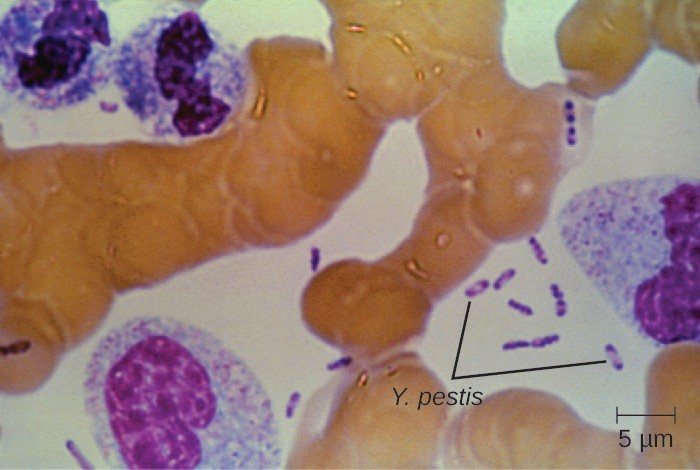
Culturing and direct microscopic examination of a sample of fluid from a bubo, blood, or sputum is the best way to identify Y. pestis and confirm a presumptive diagnosis of plague. Specimens may be stained using either a Gram, Giemsa, Wright, or Wayson’s staining technique (Figure 20.8). The bacteria show a characteristic bipolar staining pattern, resembling safety pins, that facilitates presumptive identification. Direct fluorescent antibody tests (rapid test of outer-membrane antigens) and serological tests like ELISA can be used to confirm the diagnosis. The confirmatory method for identifying Y. pestis isolates in the US is bacteriophage lysis.
Prompt antibiotic therapy can resolve most cases of bubonic plague, but septicemic and pneumonic plague are more difficult to treat because of their shorter incubation stages. Survival often depends on an early and accurate diagnosis and an appropriate choice of antibiotic therapy. In the US, the most common antibiotics used to treat patients with plague are gentamicin, fluoroquinolones, streptomycin, levofloxacin, ciprofloxacin, and doxycycline.
![]()
- Compare bubonic plague, septicemic plague, and pneumonic plague.
Micro Connections
The History of the Plague
The first recorded pandemic of plague, the Justinian plague, occurred in the sixth century CE. It is thought to have originated in central Africa and spread to the Mediterranean through trade routes. At its peak, more than 5,000 people died per day in Constantinople alone. Ultimately, one-third of that city’s population succumbed to plague.[1] The impact of this outbreak probably contributed to the later fall of Emperor Justinian. The second major pandemic, dubbed the Black Death, occurred during the 14th century. This time, the infections are thought to have originated somewhere in Asia before being transported to Europe by trade, soldiers, and war refugees. This outbreak killed an estimated one-quarter of the population of Europe (25 million, primarily in major cities). In addition, at least another 25 million are thought to have been killed in Asia and Africa.[2] This second pandemic, associated with strain Yersinia pestis biovar Medievalis, cycled for another 300 years in Europe and Great Britain, and was called the Great Plague in the 1660s.The most recent pandemic occurred in the 1890s with Yersinia pestis biovar Orientalis. This outbreak originated in the Yunnan province of China and spread worldwide through trade. It is at this time that plague made its way to the US. The etiologic agent of plague was discovered by Alexandre Yersin (1863–1943) during this outbreak as well. The overall number of deaths was lower than in prior outbreaks, perhaps because of improved sanitation and medical support.[3] Most of the deaths attributed to this final pandemic occurred in India.
Link to Learning
Click to see a video (https://openstax.org/l/22blackdeath) describing how similar the genome of the Black Death bacterium is to today’s strains of bubonic plague.
Lyme Disease
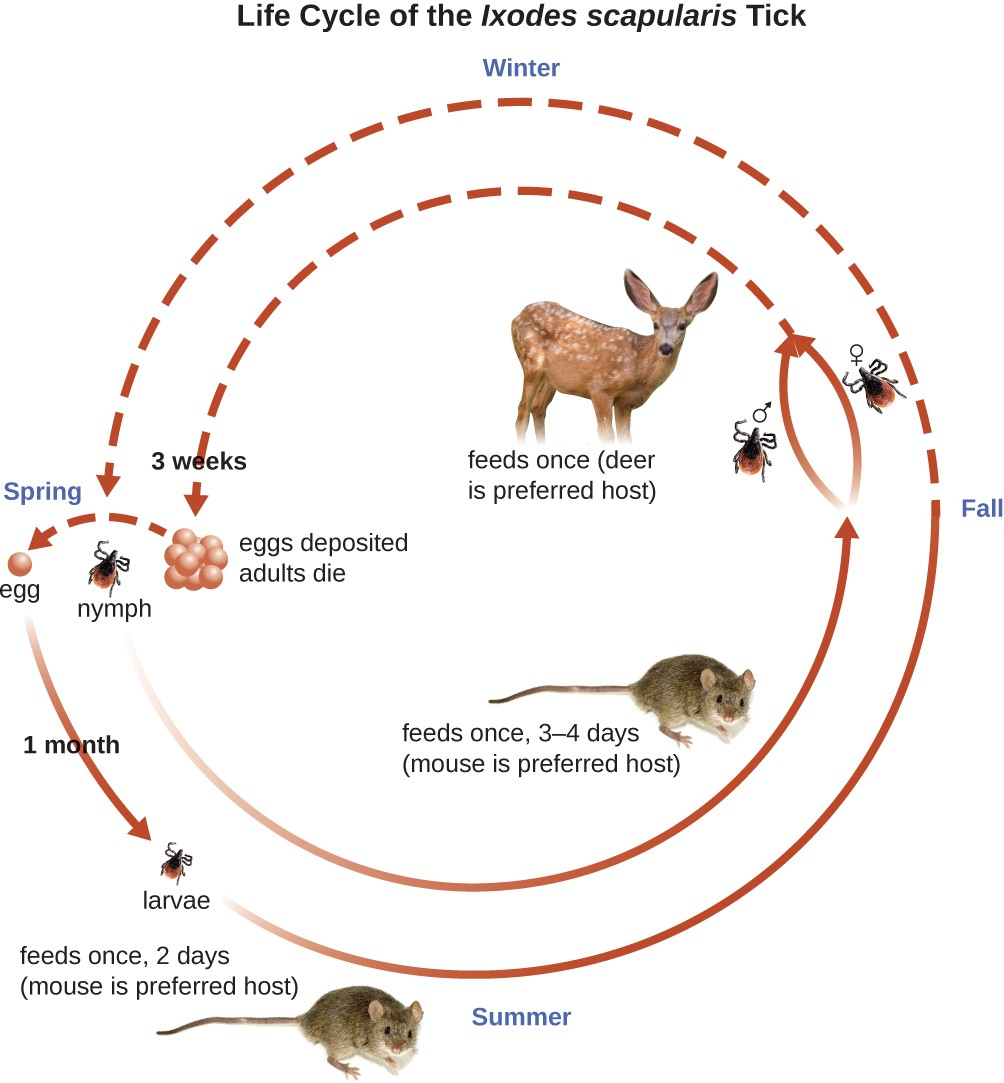
Lyme disease is caused by the spirochete Borrelia burgdorferi that is transmitted by the bite of a hard-bodied, black- legged Ixodes tick. I. scapularis is the biological vector transmitting B. burgdorferi in the eastern and north-central US and I. pacificus transmits B. burgdorferi in the western US (Figure 20.9). Different species of Ixodes ticks are responsible for B. burgdorferi transmission in Asia and Europe. In the US, Lyme disease is the most commonly reported vectorborne illness. In 2014, it was the fifth most common Nationally Notifiable disease.[4]
Ixodes ticks have complex life cycles and deer, mice, and even birds can act as reservoirs. Over 2 years, the ticks pass through four developmental stages and require a blood meal from a host at each stage. In the spring, tick eggs hatch into six-legged larvae. These larvae do not carry B. burgdorferi initially. They may acquire the spirochete when they take their first blood meal (typically from a mouse). The larvae then overwinter and molt into eight-legged nymphs in the following spring. Nymphs take blood meals primarily from small rodents, but may also feed on humans, burrowing into the skin. The feeding period can last several days to a week, and it typically takes 24 hours for an infected nymph to transmit enough B. burgdorferi to cause infection in a human host. Nymphs ultimately mature into male and female adult ticks, which tend to feed on larger animals like deer or, occasionally, humans. The adults then mate and produce eggs to continue the cycle (Figure 20.9).
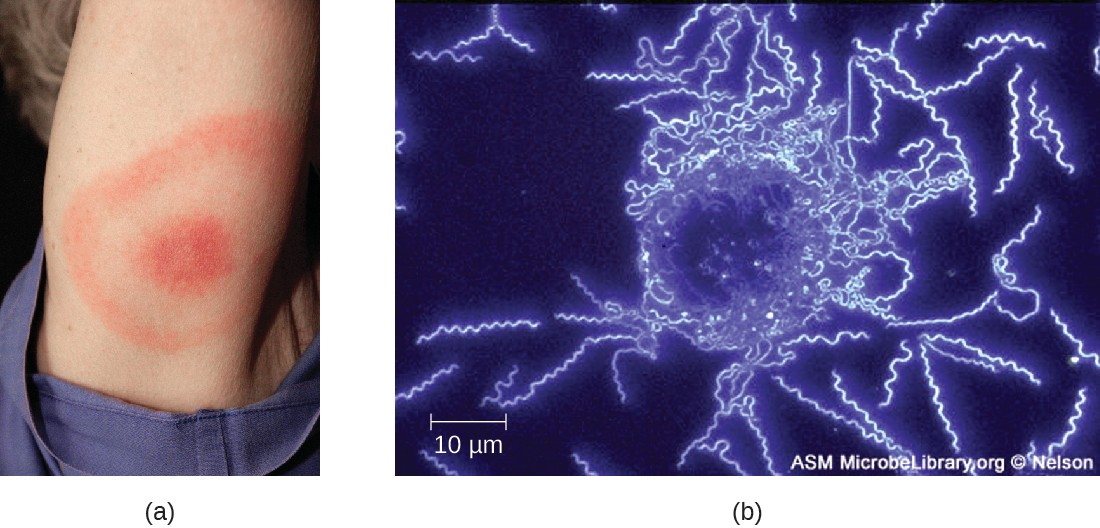
The symptoms of Lyme disease follow three stages: early localized, early disseminated, and late stage. During the early-localized stage, approximately 70%–80%[5] of cases may be characterized by a bull’s-eye rash, called erythema migrans, at the site of the initial tick bite. The rash forms 3 to 30 days after the tick bite (7 days is the average) and may also be warm to the touch (Figure 20.10).[6] This diagnostic sign is often overlooked if the tick bite occurs on the scalp or another less visible location. Other early symptoms include flu-like symptoms such as malaise, headache, fever, and muscle stiffness. If the patient goes untreated, the second early-disseminated stage of the disease occurs days to weeks later. The symptoms at this stage may include severe headache, neck stiffness, facial paralysis, arthritis, and carditis. The late-stage manifestations of the disease may occur years after exposure. Chronic inflammation causes damage that can eventually cause severe arthritis, meningitis, encephalitis, and altered mental states. The disease may be fatal if untreated.
A presumptive diagnosis of Lyme disease can be made based solely on the presence of a bull’s-eye rash at the site of infection, if it is present, in addition to other associated symptoms (Figure 20.10). In addition, indirect immunofluorescent antibody (IFA) labeling can be used to visualize bacteria from blood or skin biopsy specimens. Serological tests like ELISA can also be used to detect serum antibodies produced in response to infection. During the early stage of infection (about 30 days), antibacterial drugs such as amoxicillin and doxycycline are effective. In the later stages, penicillin G, chloramphenicol, or ceftriaxone can be given intravenously.
![]()
- What is the vector associated with epidemic typhus?
- Describe the life cycle of the deer tick and how it spreads Lyme disease.
Micro Connections
Tick Tips
Many of the diseases covered in this chapter involve arthropod vectors. Of these, ticks are probably the most commonly encountered in the US. Adult ticks have eight legs and two body segments, the cephalothorax and the head. They typically range from 2 mm to 4 mm in length, and feed on the blood of the host by attaching themselves to the skin. Unattached ticks should be removed and eliminated as soon as they are discovered. When removing a tick that has already attached itself, keep the following guidelines in mind to reduce the chances of exposure to pathogens:
- Use blunt tweezers to gently pull near the site of attachment until the tick releases its hold on the skin.
- Avoid crushing the tick’s body and do not handle the tick with bare fingers. This could release bacterial pathogens and actually increase your exposure. The tick can be killed by drowning in water or alcohol, or frozen if it may be needed later for identification and analysis.
- Disinfect the area thoroughly by swabbing with an antiseptic such as isopropanol.
- Monitor the site of the bite for rashes or other signs of infection.
Many ill-advised home remedies for tick removal have become popular in recent years, propagated by social media and pseudojournalism. Health professionals should discourage patients from resorting to any of the following methods, which are NOT recommended:
- using chemicals (e.g., petroleum jelly or fingernail polish) to dislodge an attached tick, because it can cause the tick to release fluid, which can increase the chance of infection
- using hot objects (matches or cigarette butts) to dislodge an attached tick
- squeezing the tick’s body with fingers or tweezers
Disease Profile
Bacterial Infections of the Circulatory and Lymphatic Systems
Although the circulatory system is a closed system, bacteria can enter the bloodstream through several routes. Wounds, animal bites, or other breaks in the skin and mucous membranes can result in the rapid dissemination of bacterial pathogens throughout the body. Localized infections may also spread to the bloodstream, causing serious and often fatal systemic infections. Figure 20.11 and Figure 20.12 summarize the major characteristics of bacterial infections of the circulatory and lymphatic systems.
- Rosen, William. Justinian’s Flea: Plague, Empire, and the Birth of Europe. Viking Adult; pg 3; ISBN 978-0-670-03855-8. ↵
- Benedictow, Ole J. 2004. The Black Death 1346-1353: The Complete History. Woodbridge: Boydell Press. ↵
- Centers for Disease Control and Prevention. “Plague: History.” http://www.cdc.gov/plague/history/. Accessed September 15, 2016. ↵
- Centers for Disease Control and Prevention. “Lyme Disease. Data and Statistics.” 2015. http://www.cdc.gov/lyme/stats/index.html. Accessed July 26, 2016. ↵
- Centers for Disease Control and Prevention. “Signs and Symptoms of Untreated Lyme Disease.” 2015. http://www.cdc.gov/lyme/signs_symptoms/index.html. Accessed July 27, 2016. ↵
- Centers for Disease Control and Prevention. “Ticks. Symptoms of Tickborne Illness.” 2015. http://www.cdc.gov/ticks/symptoms.html. Accessed July 27, 2016. ↵

