13.3 Cellular Defenses
Learning Objectives
- Identify and describe the components of blood
- Explain the process by which the formed elements of blood are formed (hematopoiesis)
- Describe the characteristics of formed elements found in peripheral blood, as well as their respective functions within the innate immune system
In the previous section, we discussed some of the chemical mediators found in plasma, the fluid portion of blood. The nonfluid portion of blood consists of various types of formed elements, so called because they are all formed from the same stem cells found in bone marrow. The three major categories of formed elements are: red blood cells (RBCs), also called erythrocytes; platelets, also called thrombocytes; and white blood cells (WBCs), also called leukocytes.
Red blood cells are primarily responsible for carrying oxygen to tissues. Platelets are cellular fragments that participate in blood clot formation and tissue repair. Several different types of WBCs participate in various nonspecific mechanisms of innate and adaptive immunity. In this section, we will focus primarily on the innate mechanisms of various types of WBCs.
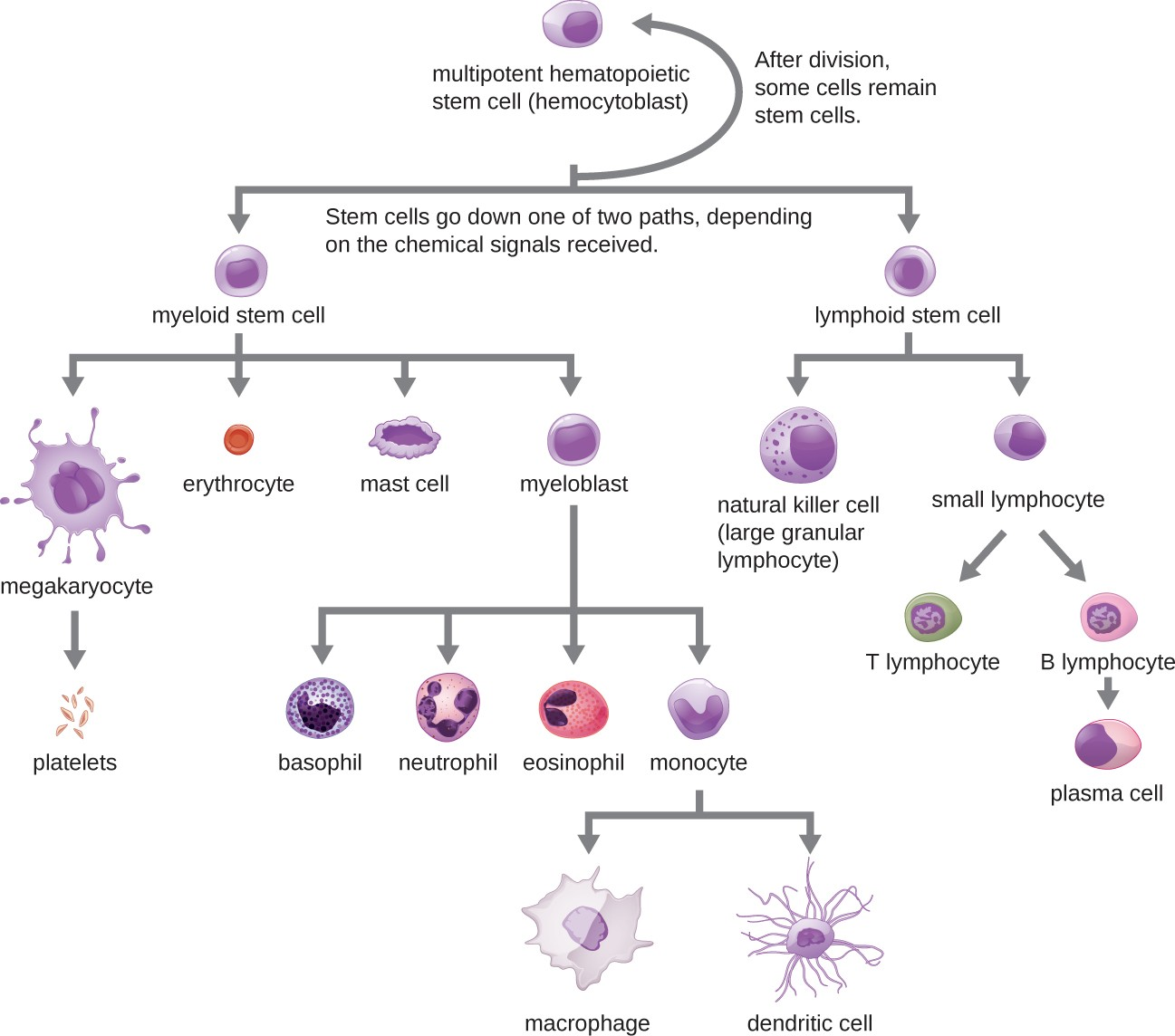
Hematopoiesis
All of the formed elements of blood are derived from pluripotent hematopoietic stem cells (HSCs) in the bone marrow. As the HSCs make copies of themselves in the bone marrow, individual cells receive different cues from the body that control how they develop and mature. As a result, the HSCs differentiate into different types of blood cells that, once mature, circulate in peripheral blood. This process of differentiation, called hematopoiesis, is shown in more detail in Figure 13.9.
In terms of sheer numbers, the vast majority of HSCs become erythrocytes. Much smaller numbers become leukocytes and platelets. Leukocytes can be further subdivided into granulocytes, which are characterized by numerous granules visible in the cytoplasm, and agranulocytes, which lack granules.
Granulocytes
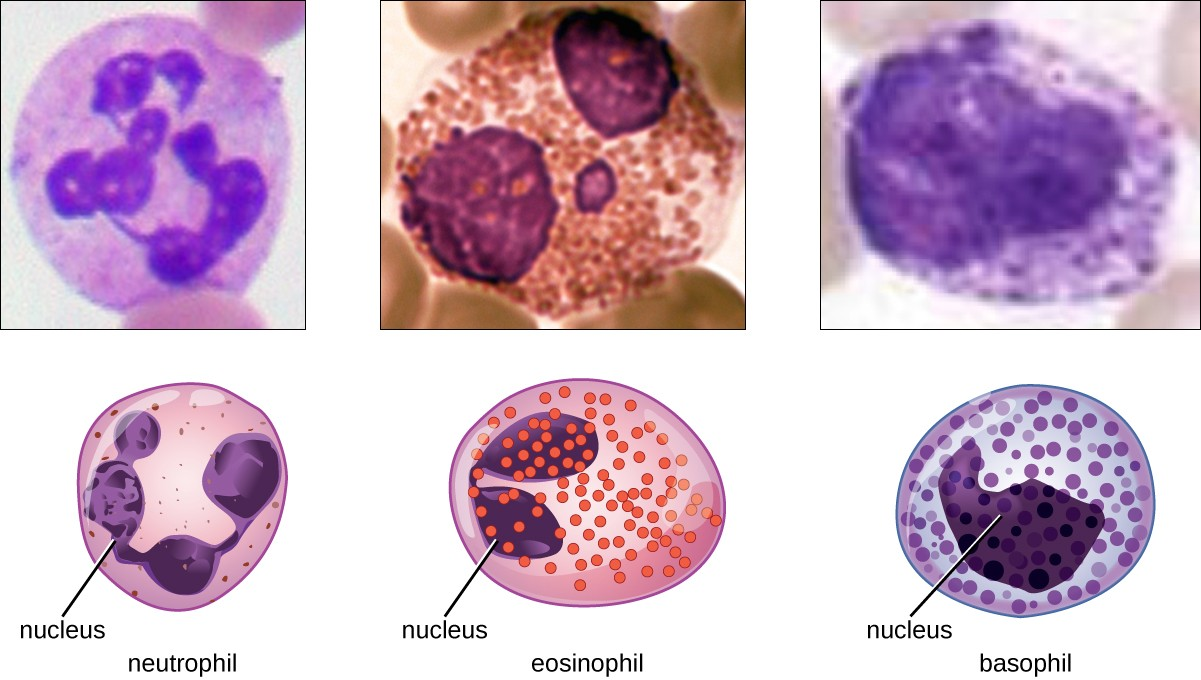
The various types of granulocytes can be distinguished from one another in a blood smear by the appearance of their nuclei and the contents of their granules, which confer different traits, functions, and staining properties. The neutrophils, also called polymorphonuclear neutrophils (PMNs), have a nucleus with three to five lobes and small, numerous, lilac-colored granules. Each lobe of the nucleus is connected by a thin strand of material to the other lobes. The eosinophils have fewer lobes in the nucleus (typically 2–3) and larger granules that stain reddish-orange. The basophils have a two-lobed nucleus and large granules that stain dark blue or purple (Figure 13.10).
Neutrophils (PMNs)
Neutrophils (PMNs) are frequently involved in the elimination and destruction of extracellular bacteria. They are capable of migrating through the walls of blood vessels to areas of bacterial infection and tissue damage, where they seek out and kill infectious bacteria. PMN granules contain a variety of defensins and hydrolytic enzymes that help them destroy bacteria through phagocytosis (described in more detail in Pathogen Recognition and Phagocytosis) In addition, when many neutrophils are brought into an infected area, they can be stimulated to release toxic molecules into the surrounding tissue to better clear infectious agents. This is called degranulation.
As neutrophils fight an infection, a visible accumulation of leukocytes, cellular debris, and bacteria at the site of infection can be observed. This buildup is what we call pus (also known as purulent or suppurative discharge or drainage). The presence of pus is a sign that the immune defenses have been activated against an infection; historically, some physicians believed that inducing pus formation could actually promote the healing of wounds. The practice of promoting “laudable pus” (by, for instance, wrapping a wound in greasy wool soaked in wine) dates back to the ancient physician Galen in the 2nd century AD, and was practiced in variant forms until the 17th century (though it was not universally accepted). Today, this method is no longer practiced because we now know that it is not effective. Although a small amount of pus formation can indicate a strong immune response, artificially inducing pus formation does not promote recovery.
Eosinophils
Eosinophils are granulocytes that protect against protozoa and helminths; they also play a role in allergic reactions. The granules of eosinophils, which readily absorb the acidic reddish dye eosin, contain histamine, degradative enzymes, and a compound known as major basic protein (MBP) (Figure 13.10). MBP binds to the surface carbohydrates of parasites, and this binding is associated with disruption of the cell membrane and membrane permeability.
Basophils
Basophils have cytoplasmic granules of varied size and are named for their granules’ ability to absorb the basic dye methylene blue (Figure 13.10). Their stimulation and degranulation can result from multiple triggering events. This cell type is important in allergic reactions and other responses that involve inflammation. One of the most abundant components of basophil granules is histamine, which is released along with other chemical factors when the basophil is stimulated. These chemicals can be chemotactic and can help to open the gaps between cells in the blood vessels.
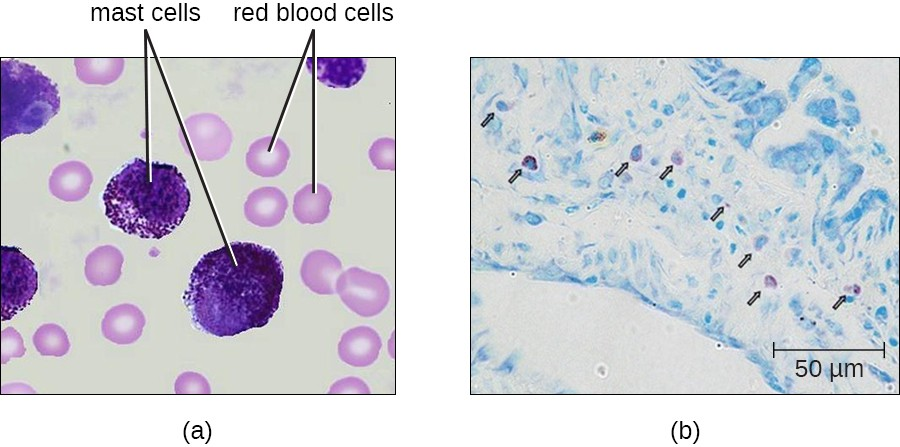
Mast Cells
Hematopoiesis also gives rise to mast cells, which appear to be derived from the same common myeloid progenitor cell as neutrophils, eosinophils, and basophils. Functionally, mast cells are very similar to basophils, containing many of the same components in their granules (e.g., histamine) and playing a similar role in allergic responses and other inflammatory reactions. However, unlike basophils, mast cells leave the circulating blood and are most frequently found residing in tissues. They are often associated with blood vessels and nerves or found close to surfaces that interface with the external environment, such as the skin and mucous membranes in various regions of the body (Figure 13.11).
![]()
- Describe the granules and nuclei of neutrophils, eosinophils, basophils, and mast cells.
- Name three antimicrobial mechanisms of neutrophils
Agranulocytes
As their name suggests, agranulocytes lack visible granules in the cytoplasm. Agranulocytes can be categorized as lymphocytes or monocytes. Among the lymphocytes are natural killer cells, which play an important role in nonspecific innate immune defenses. Lymphocytes also include the B cells and T cells, which are discussed in the next chapter because they are central players in the specific adaptive immune defenses. The monocytes differentiate into macrophages and dendritic cells, which are collectively referred to as the mononuclear phagocyte system.
Natural Killer Cells
Most lymphocytes are primarily involved in the specific adaptive immune response, and thus will be discussed in the following chapter. An exception is the natural killer cells (NK cells); these mononuclear lymphocytes use nonspecific mechanisms to recognize and destroy cells that are abnormal in some way. Cancer cells and cells infected with viruses are two examples of cellular abnormalities that are targeted by NK cells. Recognition of such cells involves a complex process of identifying inhibitory and activating molecular markers on the surface of the target cell.
Monocytes
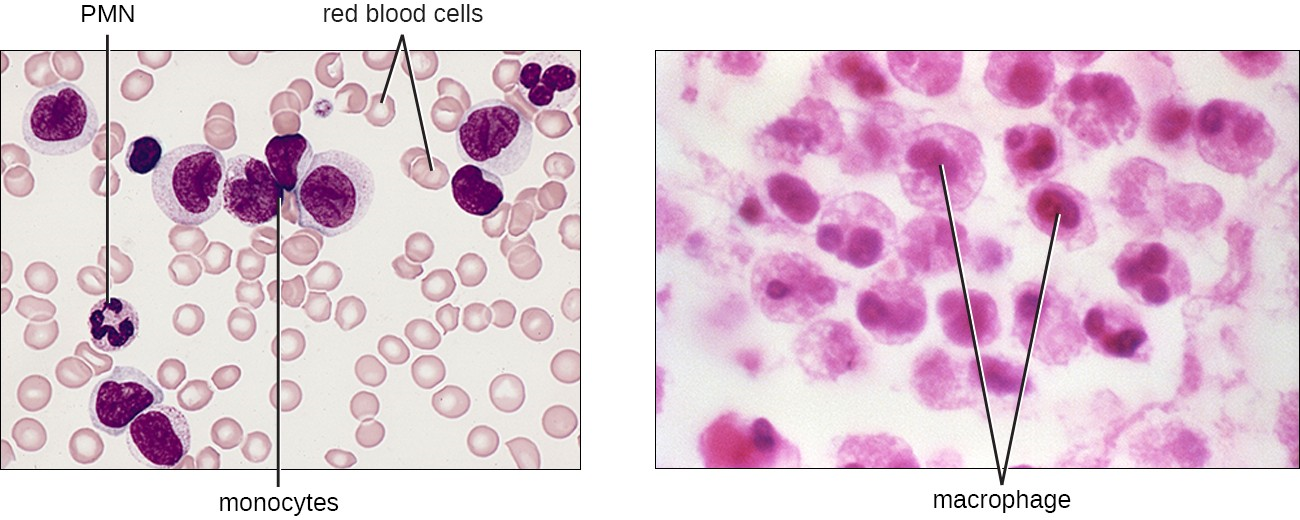
The largest of the white blood cells, monocytes have a nucleus that lacks lobes, and they also lack granules in the cytoplasm (Figure 13.12). Nevertheless, they are effective phagocytes, engulfing pathogens and apoptotic cells to help fight infection.
When monocytes leave the bloodstream and enter a specific body tissue, they differentiate into tissue-specific phagocytes called macrophages and dendritic cells. Macrophages and dendritic cells can reside in body tissues for significant lengths of time. Macrophages in specific body tissues develop characteristics suited to the particular tissue. Not only do they provide immune protection for the tissue in which they reside but they also support normal function of their neighboring tissue cells through the production of cytokines. Dendritic cells are important sentinels residing in the skin and mucous membranes, which are portals of entry for many pathogens. Monocytes, macrophages, and dendritic cells are all highly phagocytic and important promoters of the immune response through their production and release of cytokines. These cells provide an essential bridge between innate and adaptive immune responses, as discussed in the next section as well as the next chapter.
![]()
- What role do natural killer cells play for the body?
- What is the difference between monocytes and macrophages?

