13.4 Pathogen Recognition and Phagocytosis
Learning Objectives
- Explain the mechanisms by which leukocytes recognize pathogens
- Explain the process of phagocytosis and the mechanisms by which phagocytes destroy and degrade pathogens
Several of the cell types discussed in the previous section can be described as phagocytes—cells whose main function is to seek, ingest, and kill pathogens. This process, called phagocytosis, was first observed in starfish in the 1880s by Nobel Prize-winning zoologist Ilya Metchnikoff (1845–1916), who made the connection to white blood cells (WBCs) in humans and other animals. At the time, Pasteur and other scientists believed that WBCs were spreading pathogens rather than killing them (which is true for some diseases, such as tuberculosis). But in most cases, phagocytes provide a strong, swift, and effective defense against a broad range of microbes, making them a critical component of innate nonspecific immunity. This section will focus on the mechanisms by which phagocytes are able to seek, recognize, and destroy pathogens.
Pathogen Recognition
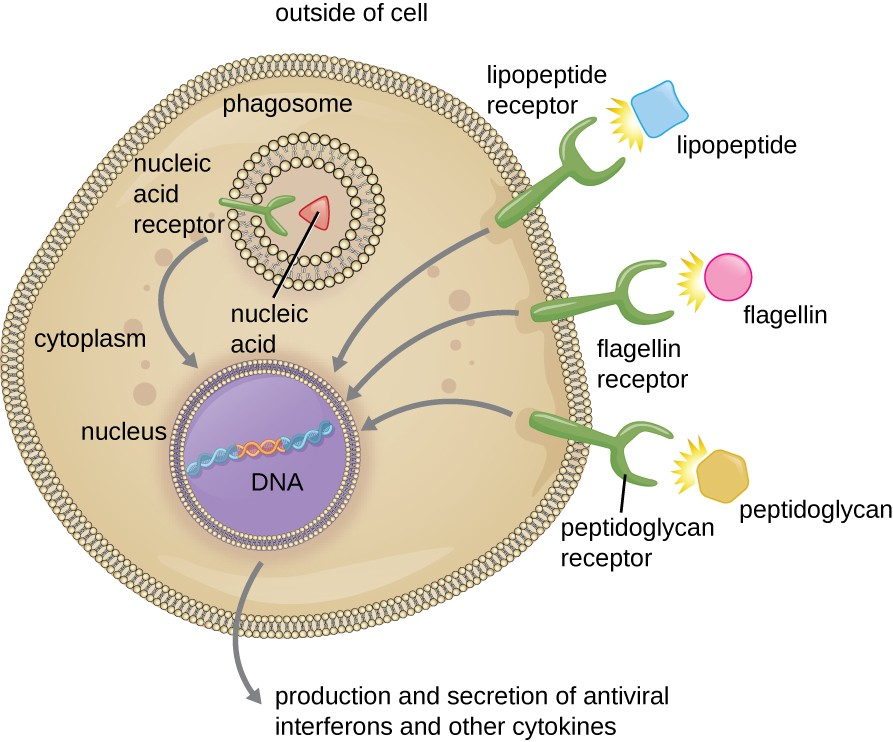
As described in the previous section, opsonization of pathogens by antibody; complement factors C1q, C3b, and C4b; and lectins can assist phagocytic cells in recognition of pathogens and attachment to initiate phagocytosis. However, not all pathogen recognition is opsonin dependent. Phagocytes can also recognize molecular structures that are common to many groups of pathogenic microbes. Such structures are called pathogen-associated molecular patterns (PAMPs). Common PAMPs include the following:
- peptidoglycan, found in bacterial cell walls;
- flagellin, a protein found in bacterial flagella;
- lipopolysaccharide (LPS) from the outer membrane of gram-negative bacteria;
- lipopeptides, molecules expressed by most bacteria; and
- nucleic acids such as viral DNA or RNA.
Like numerous other PAMPs, these substances are integral to the structure of broad classes of microbes.
The structures that allow phagocytic cells to detect PAMPs are called pattern recognition receptors (PRRs). One group of PRRs is the toll-like receptors (TLRs), which bind to various PAMPs and communicate with the nucleus of the phagocyte to elicit a response. Many TLRs (and other PRRs) are located on the surface of a phagocyte, but some can also be found embedded in the membranes of interior compartments and organelles (Figure 13.13). These interior PRRs can be useful for the binding and recognition of intracellular pathogens that may have gained access to the inside of the cell before phagocytosis could take place. Viral nucleic acids, for example, might encounter an interior PRR, triggering production of the antiviral cytokine interferon.
In addition to providing the first step of pathogen recognition, the interaction between PAMPs and PRRs on macrophages provides an intracellular signal that activates the phagocyte, causing it to transition from a dormant state of readiness and slow proliferation to a state of hyperactivity, proliferation, production/secretion of cytokines, and enhanced intracellular killing. PRRs on macrophages also respond to chemical distress signals from damaged or stressed cells. This allows macrophages to extend their responses beyond protection from infectious diseases to a broader role in the inflammatory response initiated from injuries or other diseases.
![]()
- Name four pathogen-associated molecular patterns (PAMPs).
- Describe the process of phagocyte activation.
Pathogen Degradation
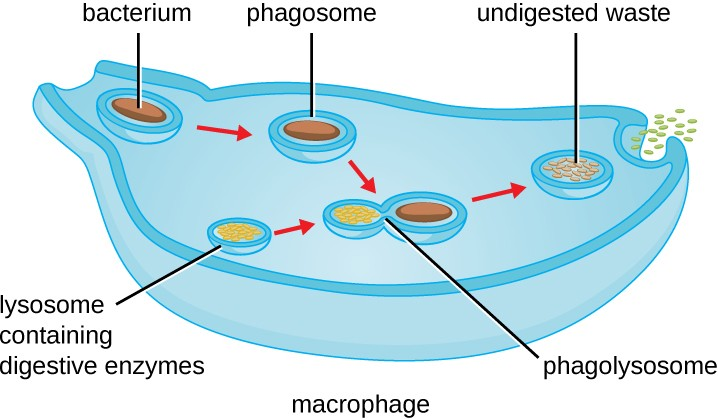
Once pathogen recognition and attachment occurs, the pathogen is engulfed in a vesicle and brought into the internal compartment of the phagocyte in a process called phagocytosis (Figure 13.14). PRRs can aid in phagocytosis by first binding to the pathogen’s surface, but phagocytes are also capable of engulfing nearby items even if they are not bound to specific receptors. To engulf the pathogen, the phagocyte forms a pseudopod that wraps around the pathogen and then pinches it off into a membrane vesicle called a phagosome. Acidification of the phagosome (pH decreases to the range of 4–5) provides an important early antibacterial mechanism. The phagosome containing the pathogen fuses with one or more lysosomes, forming a phagolysosome. Formation of the phagolysosome enhances the acidification, which is essential for activation of pH-dependent digestive lysosomal enzymes and production of hydrogen peroxide and toxic reactive oxygen species. Lysosomal enzymes such as lysozyme, phospholipase, and proteases digest the pathogen.
Once degradation is complete, leftover waste products are excreted from the cell in an exocytic vesicle. However, it is important to note that not all remains of the pathogen are excreted as waste. Macrophages and dendritic cells are also antigen-presenting cells involved in the specific adaptive immune response. These cells further process the remains of the degraded pathogen and present key antigens (specific pathogen proteins) on their cellular surface. This is an important step for stimulation of some adaptive immune responses, as will be discussed in more detail in the next chapter.
Link to Learning
Visit this link (https://openstax.org/l/22phagpathvid) to view a phagocyte chasing and engulfing a pathogen.
![]()
- What is the difference between a phagosome and a lysosome?

