17.2 Bacterial Infections of the Respiratory Tract
Learning Objectives
- Identify the most common bacteria that can cause infections of the upper and lower respiratory tract
- Compare the major characteristics of specific bacterial diseases of the respiratory tract
The respiratory tract can be infected by a variety of bacteria, both gram positive and gram negative. Although the diseases that they cause may range from mild to severe, in most cases, the microbes remain localized within the respiratory system. Fortunately, most of these infections also respond well to antibiotic therapy.
Streptococcal Infections
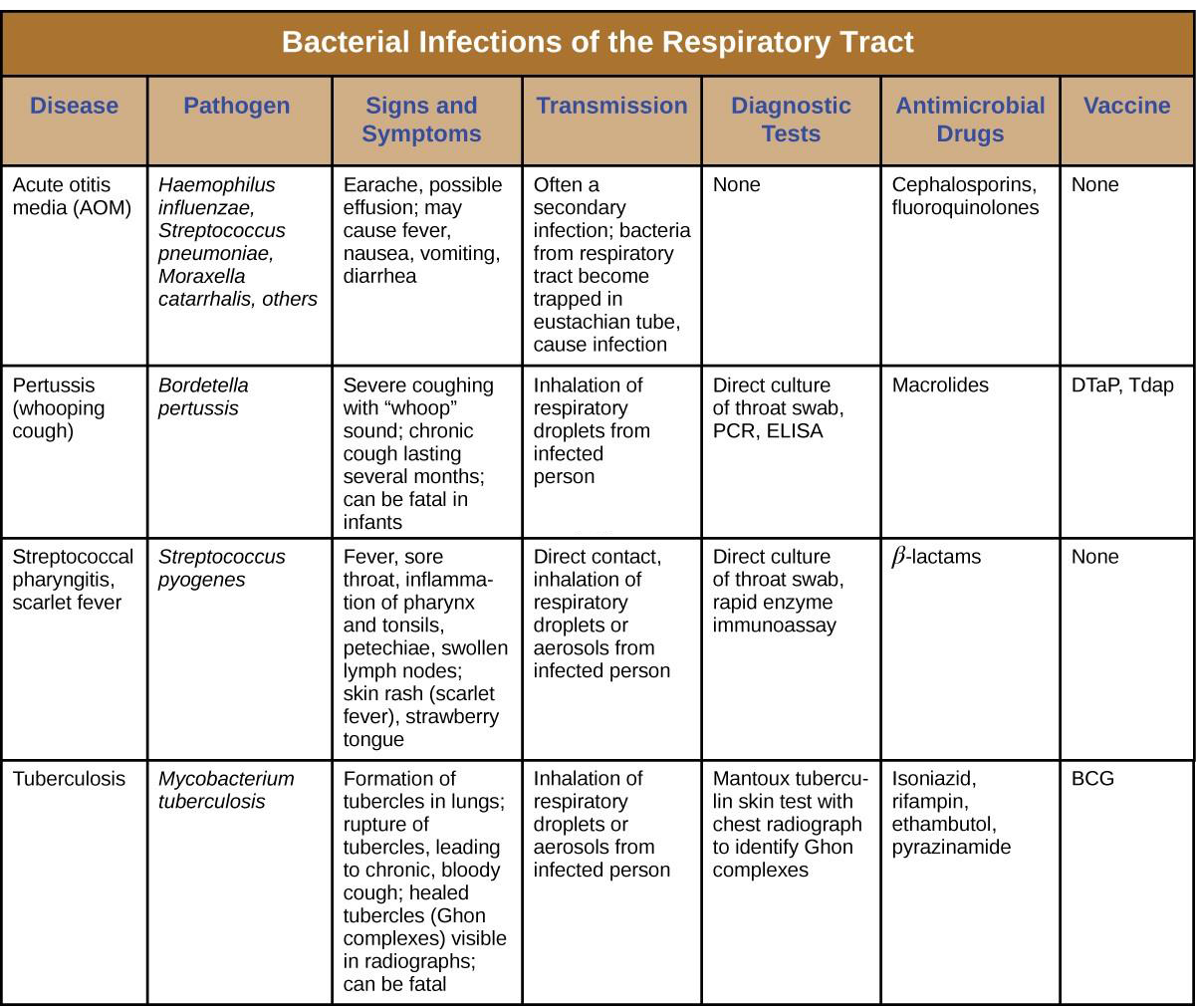
A common upper respiratory infection, streptococcal pharyngitis (strep throat) is caused by Streptococcus pyogenes. This gram-positive bacterium appears as chains of cocci, as seen in Figure 17.5. Rebecca Lancefield serologically classified streptococci in the 1930s using carbohydrate antigens from the bacterial cell walls. S. pyogenes is the sole member of the Lancefield group A streptococci and is often referred to as GAS, or group A strep.
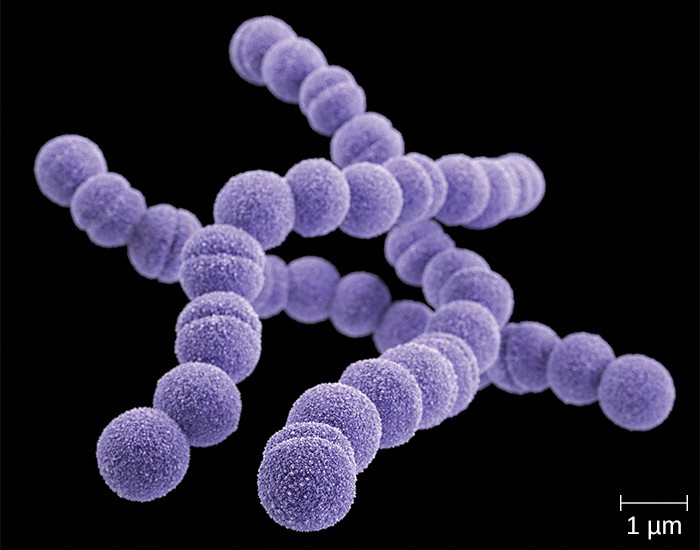
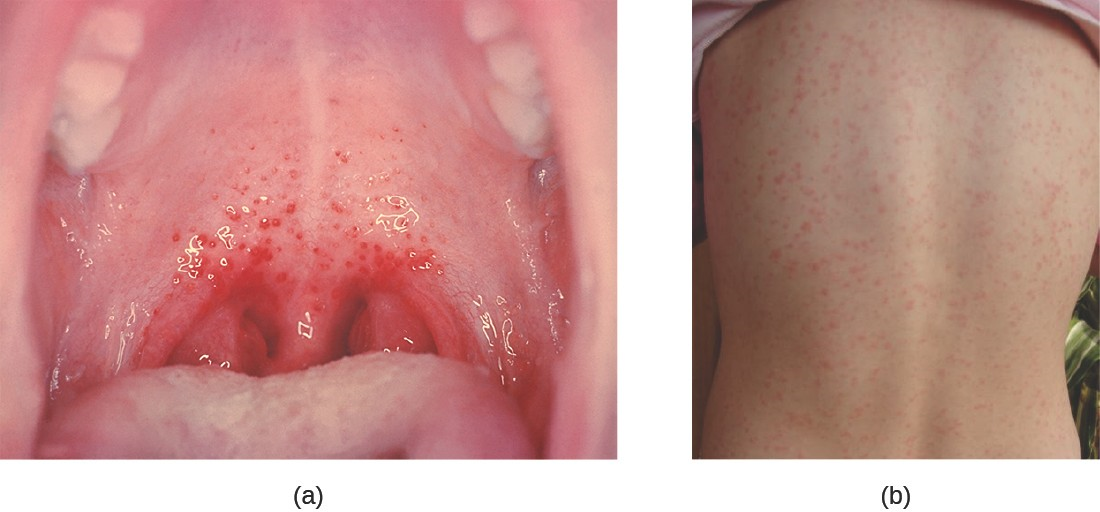
Similar to streptococcal infections of the skin, the mucosal membranes of the pharynx are damaged by the release of a variety of exoenzymes and exotoxins by this extracellular pathogen. Many strains of S. pyogenes can degrade connective tissues by using hyaluronidase, collagenase and streptokinase. Streptokinase activates plasmin, which leads to degradation of fibrin and, in turn, dissolution of blood clots, which assists in the spread of the pathogen. Released toxins include streptolysins that can destroy red and white blood cells. The classic signs of streptococcal pharyngitis are a fever higher than 38 °C (100.4 °F); intense pharyngeal pain; erythema associated with pharyngeal inflammation; and swollen, dark-red palatine tonsils, often dotted with patches of pus; and petechiae (microcapillary hemorrhages) on the soft or hard palate (roof of the mouth) (Figure 17.6). The submandibular lymph nodes beneath the angle of the jaw are also often swollen during strep throat.
Some strains of group A streptococci produce erythrogenic toxin. This exotoxin is encoded by a temperate bacteriophage (bacterial virus) and is an example of phage conversion (see The Viral Life Cycle). The toxin attacks the plasma membranes of capillary endothelial cells and leads to scarlet fever (or scarlatina), a disseminated fine red rash on the skin, and strawberry tongue, a red rash on the tongue (Figure 17.6). Severe cases may even lead tostreptococcal toxic shock syndrome (STSS), which results from massive superantigen production that leads to septic shock and death.
S. pyogenes can be easily spread by direct contact or droplet transmission through coughing and sneezing. The disease can be diagnosed quickly using a rapid enzyme immunoassay for the group A antigen. However, due to a significant rate of false-negative results (up to 30%[1]), culture identification is still the gold standard to confirm pharyngitis due to S. pyogenes. S. pyogenes can be identified as a catalase-negative, beta hemolytic bacterium that is susceptible to 0.04 units of bacitracin. Antibiotic resistance is limited for this bacterium, so most β-lactams remain effective; oral amoxicillin and intramuscular penicillin G are those most commonly prescribed.
Sequelae of S. pyogenes Infections
One reason strep throat infections are aggressively treated with antibiotics is because they can lead to serious sequelae, later clinical consequences of a primary infection. It is estimated that 1%–3% of untreated S. pyogenes infections can be followed by nonsuppurative (without the production of pus) sequelae that develop 1–3 weeks after the acute infection has resolved. Two such sequelae are acute rheumatic fever and acute glomerulonephritis.
Acute rheumatic fever can follow pharyngitis caused by specific rheumatogenic strains of S. pyogenes (strains 1, 3, 5, 6, and 18). Although the exact mechanism responsible for this sequela remains unclear, molecular mimicry between the M protein of rheumatogenic strains of S. pyogenes and heart tissue is thought to initiate the autoimmune attack. The most serious and lethal clinical manifestation of rheumatic fever is damage to and inflammation of the heart (carditis). Acute glomerulonephritis also results from an immune response to streptococcal antigens following pharyngitis and cutaneous infections. Acute glomerulonephritis develops within 6–10 days after pharyngitis, but can take up to 21 days after a cutaneous infection. Similar to acute rheumatic fever, there are strong associations between specific nephritogenic strains of S. pyogenes and acute glomerulonephritis, and evidence suggests a role for antigen mimicry and autoimmunity. However, the primary mechanism of acute glomerulonephritis appears to be the formation of immune complexes between S. pyogenes antigens and antibodies, and their deposition between endothelial cells of the glomeruli of kidney. Inflammatory response against the immune complexes leads to damage and inflammation of the glomeruli (glomerulonephritis).
![]()
- What are the symptoms of strep throat?
- What is erythrogenic toxin and what effect does it have?
- What are the causes of rheumatic fever and acute glomerulonephritis?
Acute Otitis Media
An infection of the middle ear is called acute otitis media (AOM), but often it is simply referred to as an earache. The condition is most common between ages 3 months and 3 years. In the United States, AOM is the second-leading cause of visits to pediatricians by children younger than age 5 years, and it is the leading indication for antibiotic prescription.[2]
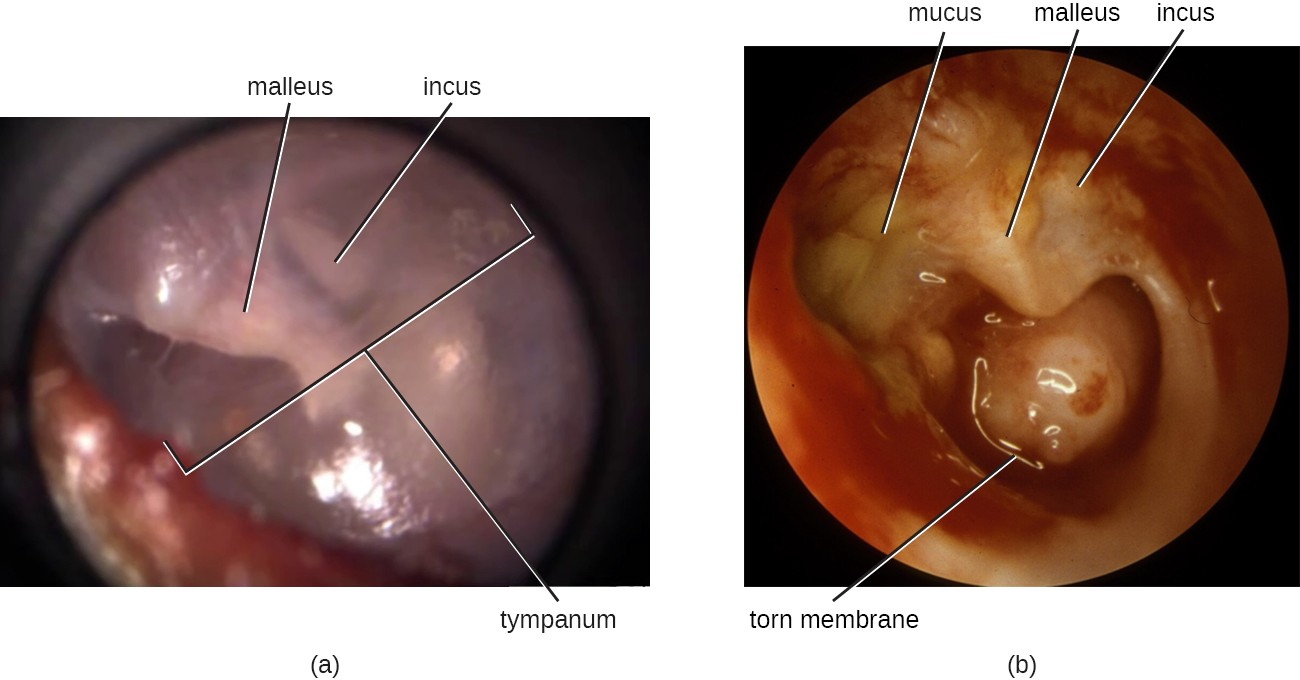
AOM is characterized by the formation and accumulation of pus in the middle ear. Unable to drain, the pus builds up, resulting in moderate to severe bulging of the tympanic membrane and otalgia (ear pain). Inflammation resulting from the infection leads to swelling of the eustachian tubes, and may also lead to fever, nausea, vomiting, and diarrhea, particularly in infants. Infants and toddlers who cannot yet speak may exhibit nonverbal signs suggesting AOM, such as holding, tugging, or rubbing of the ear, as well as uncharacteristic crying or distress in response to the pain.
AOM can be caused by a variety of bacteria. Among neonates, S. pneumoniae is the most common cause of AOM, but Escherichia coli, Enterococcus spp., and group B Streptococcus species can also be involved. In older infants and children younger than 14 years old, the most common bacterial causes are S. pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis. Among S. pneumoniae infections, encapsulated strains are frequent causes of AOM. By contrast, the strains of H. influenzae and M. cattarhalis that are responsible for AOM do not possess a capsule. Rather than direct tissue damage by these pathogens, bacterial components such as lipopolysaccharide (LPS) in gram- negative pathogens induce an inflammatory response that causes swelling, pus, and tissue damage within the middle ear (Figure 17.7).
Any blockage of the eustachian tubes, with or without infection, can cause fluid to become trapped and accumulate in the middle ear. This is referred to as otitis media with effusion (OME). The accumulated fluid offers an excellent reservoir for microbial growth and, consequently, secondary bacterial infections often ensue. This can lead to recurring and chronic earaches, which are especially common in young children. The higher incidence in children can be attributed to many factors. Children have more upper respiratory infections, in general, and their eustachian tubes are also shorter and drain at a shallower angle. Young children also tend to spend more time lying down than adults, which facilitates drainage from the nasopharynx through the eustachian tube and into the middle ear. Bottle feeding while lying down enhances this risk because the sucking action on the bottle causes negative pressure to build up within the eustachian tube, promoting the movement of fluid and bacteria from the nasopharynx.
Diagnosis is typically made based on clinical signs and symptoms, without laboratory testing to determine the specific causative agent. Antibiotics are frequently prescribed for the treatment of AOM. High-dose amoxicillin is the first-line drug, but with increasing resistance concerns, macrolides and cephalosporins may also be used. The pneumococcal conjugate vaccine (PCV13) contains serotypes that are important causes of AOM, and vaccination has been shown to decrease the incidence of AOM. Vaccination against influenza has also been shown to decrease the risk for AOM, likely because viral infections like influenza predispose patients to secondary infections with S. pneumoniae. Although there is a conjugate vaccine available for the invasive serotype B of H. influenzae, this vaccine does not impact the incidence of H. influenzae AOM. Because unencapsulated strains of H. influenzae and M. catarrhalis are involved in AOM, vaccines against bacterial cellular factors other than capsules will need to be developed.
![]()
- What are the usual causative agents of acute otitis media?
- What factors facilitate acute otitis media with effusion in young children?
Bacterial Pneumonia
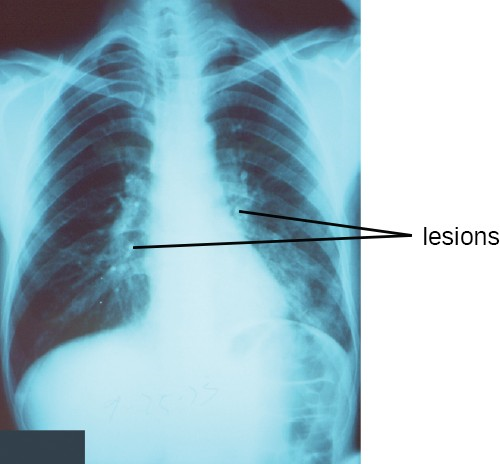
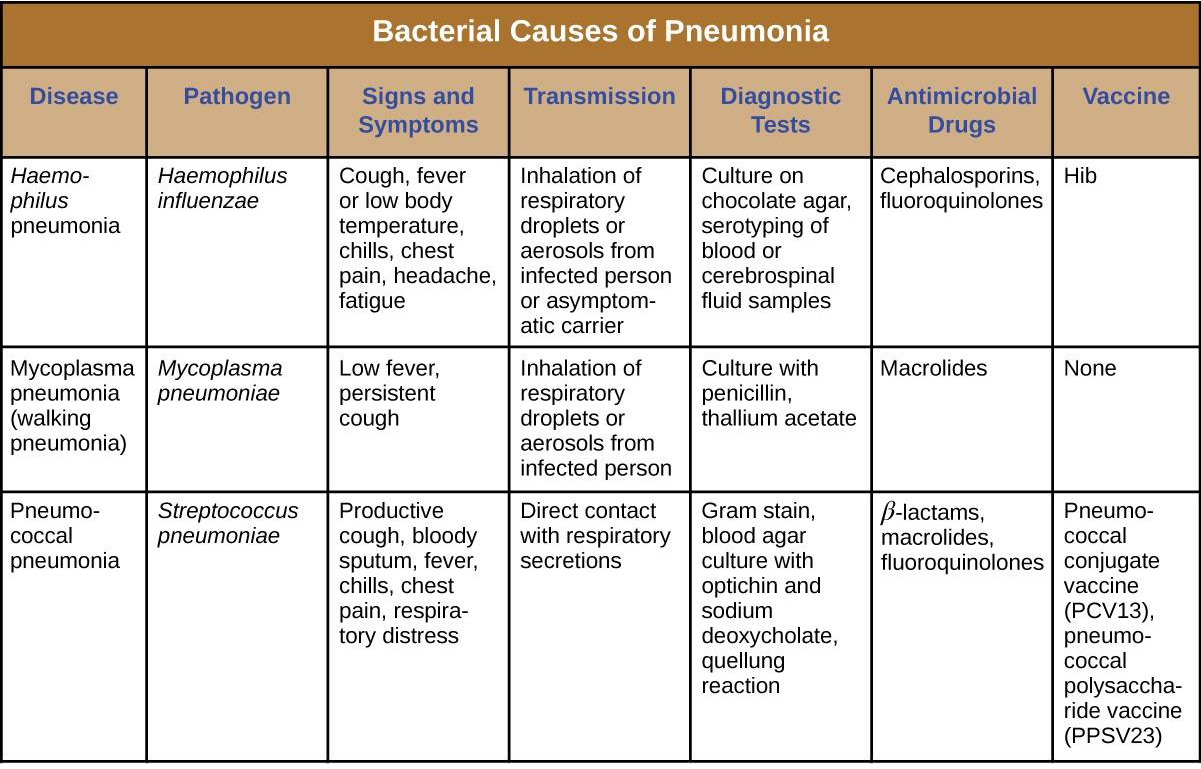
Pneumonia is a general term for infections of the lungs that lead to inflammation and accumulation of fluids and white blood cells in the alveoli. Pneumonia can be caused by bacteria, viruses, fungi, and other organisms, although the vast majority of pneumonias are bacterial in origin. Bacterial pneumonia is a prevalent, potentially serious infection; it caused more 50,000 deaths in the United States in 2014.[3] As the alveoli fill with fluids and white blood cells (consolidation), air exchange becomes impaired and patients experience respiratory distress (Figure 17.8). In addition, pneumonia can lead to pleurisy, an infection of the pleural membrane surrounding the lungs, which can make breathing very painful. Although many different bacteria can cause pneumonia under the right circumstances, three bacterial species cause most clinical cases: Streptococcus pneumoniae, H. influenzae, and Mycoplasma pneumoniae. In addition to these, we will also examine some of the less common causes of pneumonia.
Pneumococcal Pneumonia
The most common cause of community-acquired bacterial pneumonia is Streptococcus pneumoniae. This gram- positive, alpha hemolytic streptococcus is commonly found as part of the normal microbiota of the human respiratory tract. The cells tend to be somewhat lancet-shaped and typically appear as pairs (Figure 17.9). The pneumococci initially colonize the bronchioles of the lungs. Eventually, the infection spreads to the alveoli, where the microbe’s polysaccharide capsule interferes with phagocytic clearance. Other virulence factors include autolysins like Lyt A, which degrade the microbial cell wall, resulting in cell lysis and the release of cytoplasmic virulence factors. One of these factors, pneumolysin O, is important in disease progression; this pore-forming protein damages host cells, promotes bacterial adherence, and enhances pro-inflammatory cytokine production. The resulting inflammatory response causes the alveoli to fill with exudate rich in neutrophils and red blood cells. As a consequence, infected individuals develop a productive cough with bloody sputum.
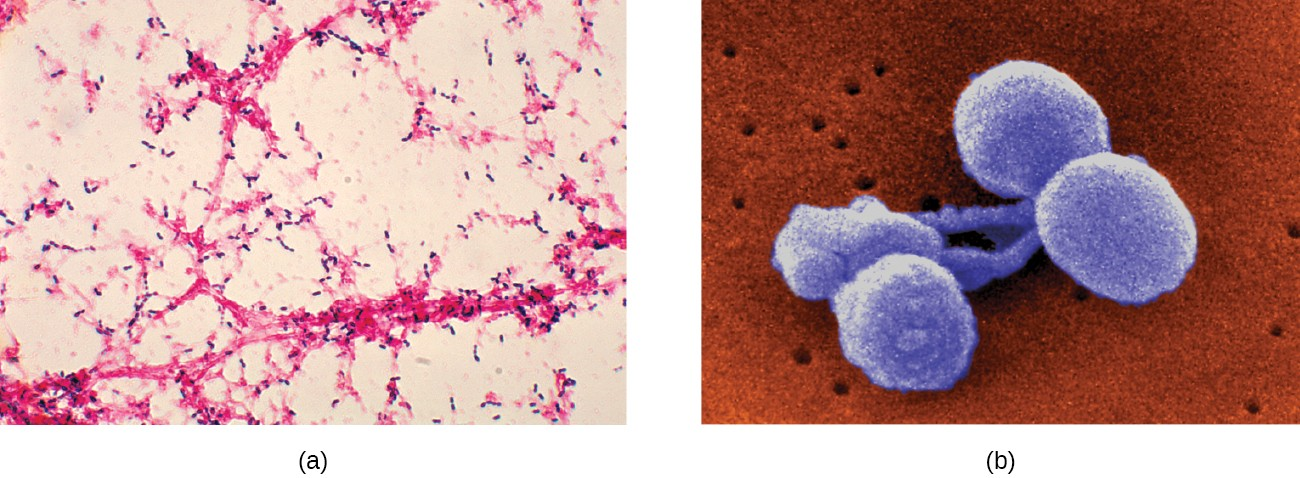
Pneumococci can be presumptively identified by their distinctive gram-positive, lancet-shaped cell morphology and diplococcal arrangement. In blood agar cultures, the organism demonstrates alpha hemolytic colonies that are autolytic after 24 to 48 hours. In addition, S. pneumoniae is extremely sensitive to optochin and colonies are rapidly destroyed by the addition of 10% solution of sodium deoxycholate. All clinical pneumococcal isolates are serotyped using the quellung reaction with typing antisera produced by the CDC. Positive quellung reactions are considered definitive identification of pneumococci.
Antibiotics remain the mainstay treatment for pneumococci. β-Lactams like penicillin are the first-line drugs, but resistance to β-lactams is a growing problem. When β-lactam resistance is a concern, macrolides and fluoroquinolones may be prescribed. However, S. pneumoniae resistance to macrolides and fluoroquinolones is increasing as well, limiting the therapeutic options for some infections. There are currently two pneumococcal vaccines available: pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23). These are generally given to the most vulnerable populations of individuals: children younger than 2 years and adults older than 65 years.
Haemophilus Pneumonia
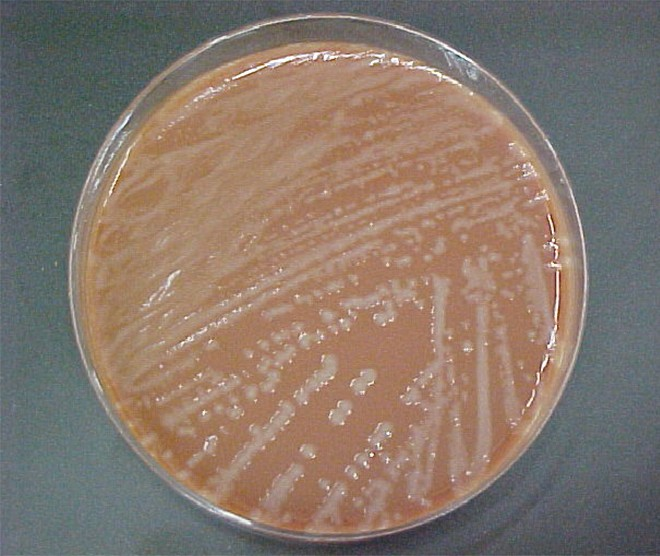
Encapsulated strains of Haemophilus influenzae are known for causing meningitis, but nonencapsulated strains are important causes of pneumonia. This small, gram-negative coccobacillus is found in the pharynx of the majority of healthy children; however, Haemophilus pneumonia is primarily seen in the elderly. Like other pathogens that cause pneumonia, H. influenzae is spread by droplets and aerosols produced by coughing. A fastidious organism, H. influenzae will only grow on media with available factor X (hemin) and factor V (NAD), like chocolate agar (Figure 17.10). Serotyping must be performed to confirm identity of H. influenzae isolates.
Infections of the alveoli by H. influenzae result in inflammation and accumulation of fluids. Increasing resistance to β-lactams, macrolides, and tetracyclines presents challenges for the treatment of Haemophilus pneumonia. Resistance to the fluoroquinolones is rare among isolates of H. influenzae but has been observed. As discussed for AOM, a vaccine directed against nonencapsulated H. influenzae, if developed, would provide protection against pneumonia caused by this pathogen.
Mycoplasma Pneumonia (Walking Pneumonia)
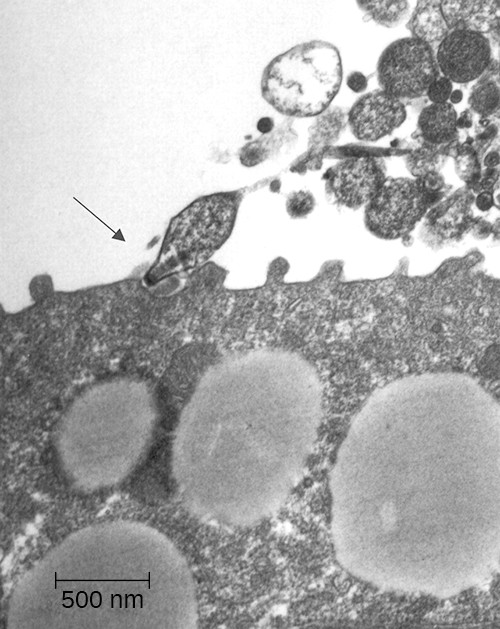
Primary atypical pneumonia is caused by Mycoplasma pneumoniae. This bacterium is not part of the respiratory tract’s normal microbiota and can cause epidemic disease outbreaks. Also known as walking pneumonia, mycoplasma pneumonia infections are common in crowded environments like college campuses and military bases. It is spread by aerosols formed when coughing or sneezing. The disease is often mild, with a low fever and persistent cough. These bacteria, which do not have cell walls, use a specialized attachment organelle to bind to ciliated cells. In the process, epithelial cells are damaged and the proper function of the cilia is hindered (Figure 17.11).
Case in Point
Why Me?
Tracy is a 6-year old who developed a serious cough that would not seem to go away. After 2 weeks, her parents became concerned and took her to the pediatrician, who suspected a case of bacterial pneumonia. Tests confirmed that the cause was Haemophilus influenzae. Fortunately, Tracy responded well to antibiotic treatment and eventually made a full recovery.
Because there had been several other cases of bacterial pneumonia at Tracy’s elementary school, local health officials urged parents to have their children screened. Of the children who were screened, it was discovered that greater than 50% carried H. influenzae in their nasal cavities, yet all but two of them were asymptomatic.
Why is it that some individuals become seriously ill from bacterial infections that seem to have little or no effect on others? The pathogenicity of an organism—its ability to cause host damage—is not solely a property of the microorganism. Rather, it is the product of a complex relationship between the microbe’s virulence factors and the immune defenses of the individual. Preexisting conditions and environmental factors such as exposure to secondhand smoke can make some individuals more susceptible to infection by producing conditions favorable to microbial growth or compromising the immune system. In addition, individuals may have genetically determined immune factors that protect them—or not—from particular strains of pathogens. The interactions between these host factors and the pathogenicity factors produced by the microorganism ultimately determine the outcome of the infection. A clearer understanding of these interactions may allow for better identification of at-risk individuals and prophylactic interventions in the future.
Mycoplasma grow very slowly when cultured. Therefore, penicillin and thallium acetate are added to agar to prevent the overgrowth by faster-growing potential contaminants. Since M. pneumoniae does not have a cell wall, it is resistant to these substances. Without a cell wall, the microbial cells appear pleomorphic. M. pneumoniae infections tend to be self-limiting but may also respond well to macrolide antibiotic therapy. β-lactams, which target cell wall synthesis, are not indicated for treatment of infections with this pathogen.
![]()
- What three pathogens are responsible for the most prevalent types of bacterial pneumonia?
- Which cause of pneumonia is most likely to affect young people?
Tuberculosis
Tuberculosis (TB) is one of the deadliest infectious diseases in human history. Although tuberculosis infection rates in the United States are extremely low, the CDC estimates that about one-third of the world’s population is infected with Mycobacterium tuberculosis, the causal organism of TB, with 9.6 million new TB cases and 1.5 million deaths worldwide in 2014.[4]
M. tuberculosis is an acid-fast, high G + C, gram-positive, nonspore-forming rod. Its cell wall is rich in waxy mycolic acids, which make the cells impervious to polar molecules. It also causes these organisms to grow slowly.
M. tuberculosis causes a chronic granulomatous disease that can infect any area of the body, although it is typically associated with the lungs. M. tuberculosis is spread by inhalation of respiratory droplets or aerosols from an infected person. The infectious dose of M. tuberculosis is only 10 cells.[5]
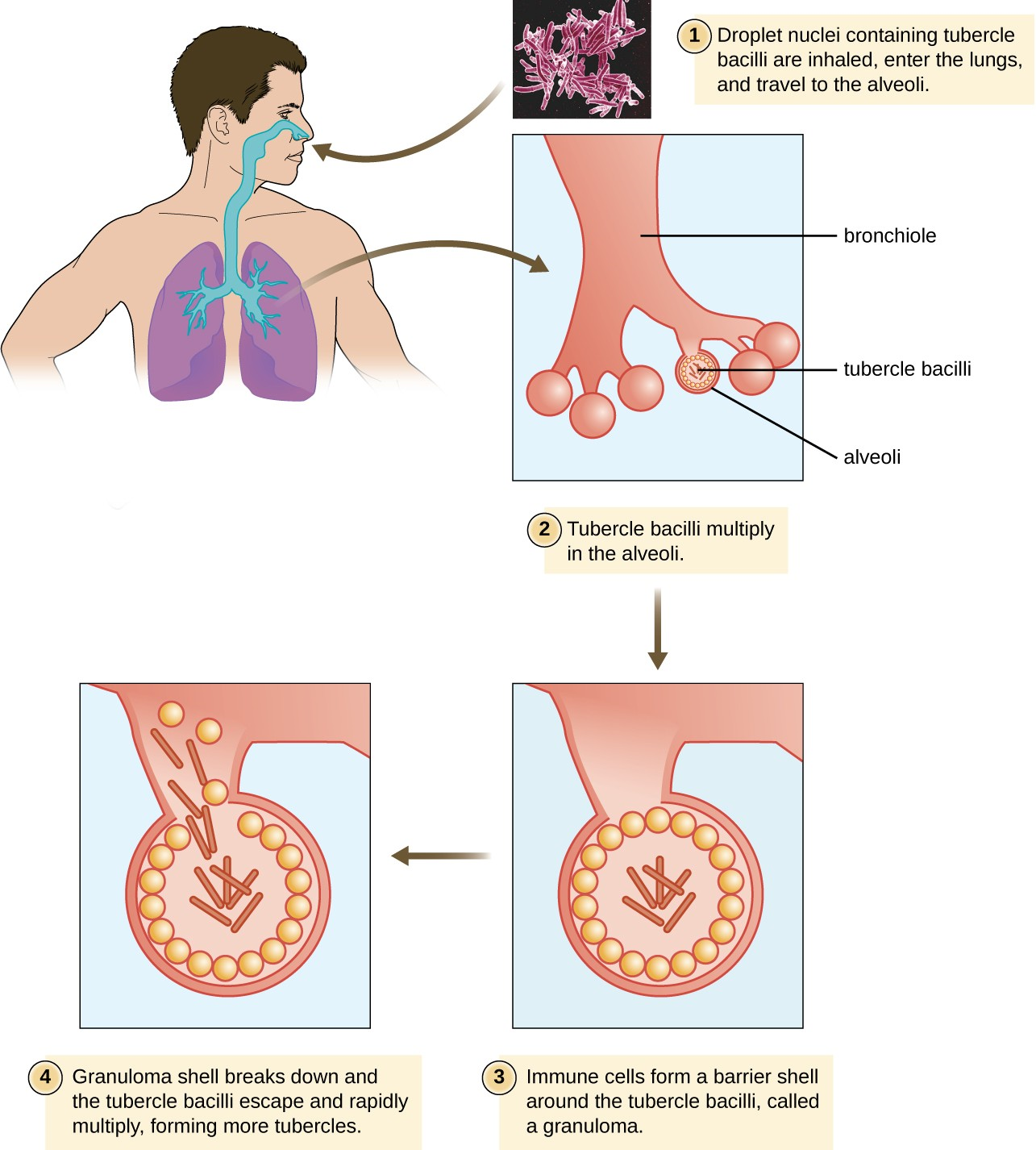
After inhalation, the bacteria enter the alveoli (Figure 17.12). The cells are phagocytized by macrophages but can survive and multiply within these phagocytes because of the protection by the waxy mycolic acid in their cell walls. If not eliminated by macrophages, the infection can progress, causing an inflammatory response and an accumulation of neutrophils and macrophages in the area. Several weeks or months may pass before an immunological response is mounted by T cells and B cells. Eventually, the lesions in the alveoli become walled off, forming small round lesions called tubercles. Bacteria continue to be released into the center of the tubercles and the chronic immune response results in tissue damage and induction of apoptosis (programmed host-cell death) in a process called liquefaction. This creates a caseous center, or air pocket, where the aerobic M. tuberculosis can grow and multiply. Tubercles may eventually rupture and bacterial cells can invade pulmonary capillaries; from there, bacteria can spread through the bloodstream to other organs, a condition known as miliary tuberculosis. The rupture of tubercles also facilitates transmission of the bacteria to other individuals via droplet aerosols that exit the body in coughs. Because these droplets can be very small and stay aloft for a long time, special precautions are necessary when caring for patients with TB, such as the use of face masks and negative-pressure ventilation and filtering systems.
Eventually, most lesions heal to form calcified Ghon complexes. These structures are visible on chest radiographs and are a useful diagnostic feature. But even after the disease has apparently ended, viable bacteria remain sequestered in these locations. Release of these organisms at a later time can produce reactivation tuberculosis (or secondary TB). This is mainly observed in people with alcoholism, the elderly, or in otherwise immunocompromised individuals (Figure 17.12).
Because TB is a chronic disease, chemotherapeutic treatments often continue for months or years. Multidrug resistant (MDR-TB) and extensively drug-resistant (XDR-TB) strains of M. tuberculosis are a growing clinical concern. These strains can arise due to misuse or mismanagement of antibiotic therapies. Therefore, it is imperative that proper multidrug protocols are used to treat these infections. Common antibiotics included in these mixtures are isoniazid, rifampin, ethambutol, and pyrazinamide.
A TB vaccine is available that is based on the so-called bacillus Calmette-Guérin (BCG) strain of M. bovis commonly found in cattle. In the United States, the BCG vaccine is only given to health-care workers and members of the military who are at risk of exposure to active cases of TB. It is used more broadly worldwide. Many individuals born in other countries have been vaccinated with BCG strain. BCG is used in many countries with a high prevalence of TB, to prevent childhood tuberculous meningitis and miliary disease.
FS was here
The Mantoux tuberculin skin test (Figure 17.13) is regularly used in the United States to screen for potential TB exposure (see Hypersensitivities). However, prior vaccinations with the BCG vaccine can cause false-positive results. Chest radiographs to detect Ghon complex formation are required, therefore, to confirm exposure.
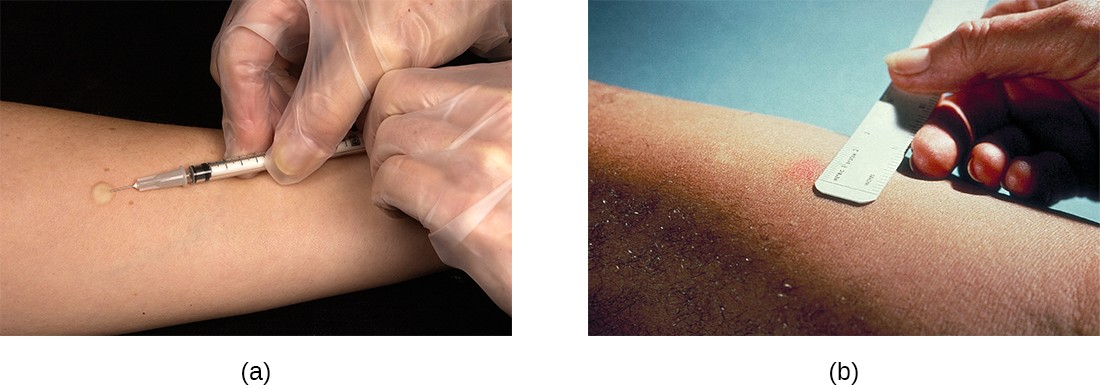
Link to Learning
These short animations (https://openstax.org/l/22mycotublegpnean) discuss the infection strategies of Mycobacterium tuberculosis and Legionella pneumophila.
![]()
- What characteristic of Mycobacterium tuberculosis allows it to evade the immune response?
- What happens to cause miliary tuberculosis?
- Explain the limitations of the Mantoux tuberculin skin test.
Pertussis (Whooping Cough)
The causative agent of pertussis, commonly called whooping cough, is Bordetella pertussis, a gram-negative coccobacillus. The disease is characterized by mucus accumulation in the lungs that leads to a long period of severe coughing. Sometimes, following a bout of coughing, a sound resembling a “whoop” is produced as air is inhaled through the inflamed and restricted airway—hence the name whooping cough. Although adults can be infected, the symptoms of this disease are most pronounced in infants and children. Pertussis is highly communicable through droplet transmission, so the uncontrollable coughing produced is an efficient means of transmitting the disease in a susceptible population.
Following inhalation, B. pertussis specifically attaches to epithelial cells using an adhesin, filamentous hemagglutinin. The bacteria then grow at the site of infection and cause disease symptoms through the production of exotoxins. One of the main virulence factors of this organism is an A-B exotoxin called the pertussis toxin (PT). When PT enters the host cells, it increases the cyclic adenosine monophosphate (cAMP) levels and disrupts cellular signaling. PT is known to enhance inflammatory responses involving histamine and serotonin. In addition to PT, B. pertussis produces a tracheal cytotoxin that damages ciliated epithelial cells and results in accumulation of mucus in the lungs. The mucus can support the colonization and growth of other microbes and, as a consequence, secondary infections are common. Together, the effects of these factors produce the cough that characterizes this infection.
A pertussis infection can be divided into three distinct stages. The initial infection, termed the catarrhal stage, is relatively mild and unremarkable. The signs and symptoms may include nasal congestion, a runny nose, sneezing, and a low-grade fever. This, however, is the stage in which B. pertussis is most infectious. In the paroxysmal stage, mucus accumulation leads to uncontrollable coughing spasms that can last for several minutes and frequently induce vomiting. The paroxysmal stage can last for several weeks. A long convalescence stage follows the paroxysmal stage, during which time patients experience a chronic cough that can last for up to several months. In fact, the disease is sometimes called the 100-day cough.
In infants, coughing can be forceful enough to cause fractures to the ribs, and prolonged infections can lead to death. The CDC reported 20 pertussis-related deaths in 2012,[7] but that number had declined to five by 2015.[8]
During the first 2 weeks of infection, laboratory diagnosis is best performed by culturing the organism directly from a nasopharyngeal (NP) specimen collected from the posterior nasopharynx. The NP specimen is streaked onto Bordet- Gengou medium. The specimens must be transported to the laboratory as quickly as possible, even if transport media are used. Transport times of longer than 24 hours reduce the viability of B. pertussis significantly.
Pertussis is generally a self-limiting disease. Antibiotic therapy with erythromycin or tetracycline is only effective at the very earliest stages of disease. Antibiotics given later in the infection, and prophylactically to uninfected individuals, reduce the rate of transmission. Active vaccination is a better approach to control this disease. The DPT vaccine was once in common use in the United States. In that vaccine, the P component consisted of killed whole-cell B. pertussis preparations. Because of some adverse effects, that preparation has now been superseded by the DTaP and Tdap vaccines. In both of these new vaccines, the “aP” component is a pertussis toxoid.
Widespread vaccination has greatly reduced the number of reported cases and prevented large epidemics of pertussis. Recently, however, pertussis has begun to reemerge as a childhood disease in some states because of declining vaccination rates and an increasing population of susceptible children.
Link to Learning
This webpage (https://openstax.org/l/22pertussaudio) contains an audio clip of the distinctive “whooping” sound associated with pertussis in infants.
This interactive map (https://openstax.org/l/22intmapprevacc) shows outbreaks of vaccine preventable diseases, including pertussis, around the world.
![]()
- What accounts for the mucus production in a pertussis infection?
- What are the signs and symptoms associated with the three stages of pertussis?
- Why is pertussis becoming more common in the United States?
- WL Lean et al. “Rapid Diagnostic Tests for Group A Streptococcal Pharyngitis: A Meta-Analysis.” Pediatrics 134, no. 4 (2014):771–781. ↵
- G. Worrall. “Acute Otitis Media.” Canadian Family Physician 53 no. 12 (2007):2147–2148. ↵
- KD Kochanek et al. “Deaths: Final Data for 2014.” National Vital Statistics Reports 65 no 4 (2016). ↵
- Centers for Disease Control and Prevention. “Tuberculosis (TB). Data and Statistics.” http://www.cdc.gov/tb/statistics/default.htm ↵
- D. Saini et al. “Ultra-Low Dose of Mycobacterium tuberculosis Aerosol Creates Partial Infection in Mice.” Tuberculosis 92 no. 2 (2012):160–165. ↵
- G. Kaplan et al. “Mycobacterium tuberculosis Growth at the Cavity Surface: A Microenvironment with Failed Immunity.” Infection and Immunity 71 no.12 (2003):7099–7108. ↵
- Centers for Disease Control and Prevention. “2012 Final Pertussis Surveillance Report.” 2015. http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2012.pdf. Accessed July 6, 2016. ↵
- Centers for Disease Control and Prevention. “2015 Provisional Pertussis Surveillance Report.” 2016. http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2015-provisional.pdf. Accessed July 6, 2016. ↵

