Main Body
Integration of Macronutrient & Alcohol Metabolism
Understanding the different metabolic pathways is an important step. However, an integrated understanding of the interconnectedness and tissue specificity of metabolism is where this knowledge really becomes powerful. To this end, we will first cover how the different pathways feed into one another and then talk about the metabolic capabilities of the different tissues in the body. We will then discuss what happens metabolically during different conditions or when consuming certain diets.
Sections:
7.1 Integration of Macronutrient & Alcohol Metabolic Pathways
7.2 Liver Macronutrient & Alcohol Metabolism
7.3 Extrahepatic Macronutrient & Alcohol Metabolism
7.4 Metabolic Conditions
7.1 Integration of Macronutrient and Alcohol Metabolic Pathways
If you were to draw all the macronutrient and alcohol metabolic pathways covered in chapter 6, hopefully it would look something like the figure below. In this figure:
Carbohydrate pathways are orange
Triglyceride/fatty acid pathways are purple
Protein/amino acid pathways are green
Nonclassified pathways are gray
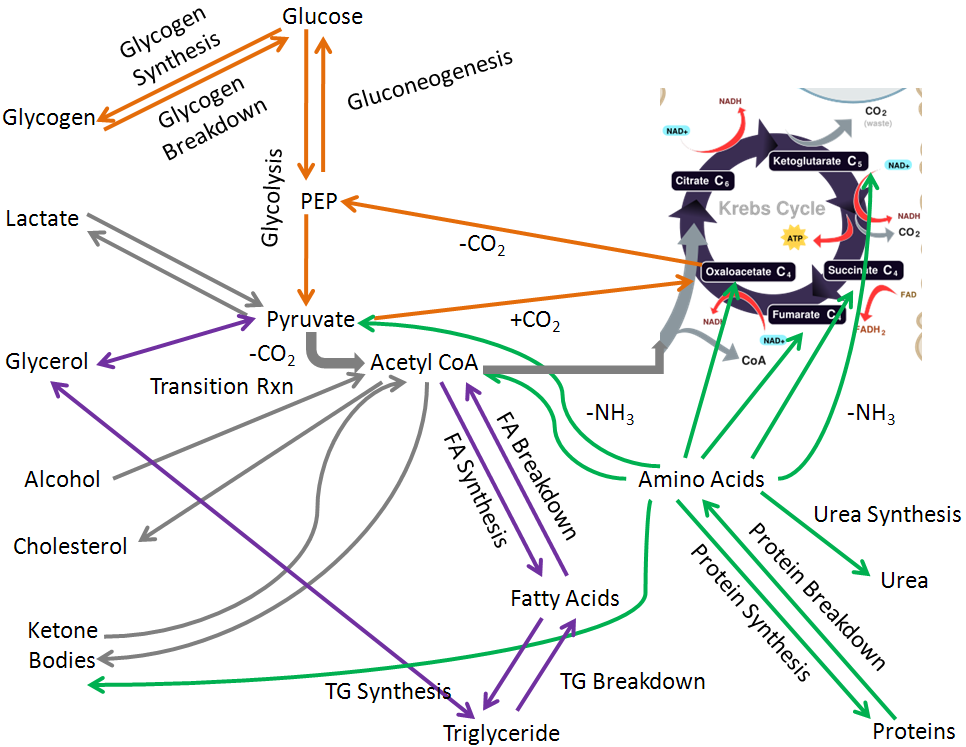
To simplify, we are going to remove the glycerol and cholesterol pathways so that we can focus on integrating the other pathways in macronutrient and alcohol metabolism.
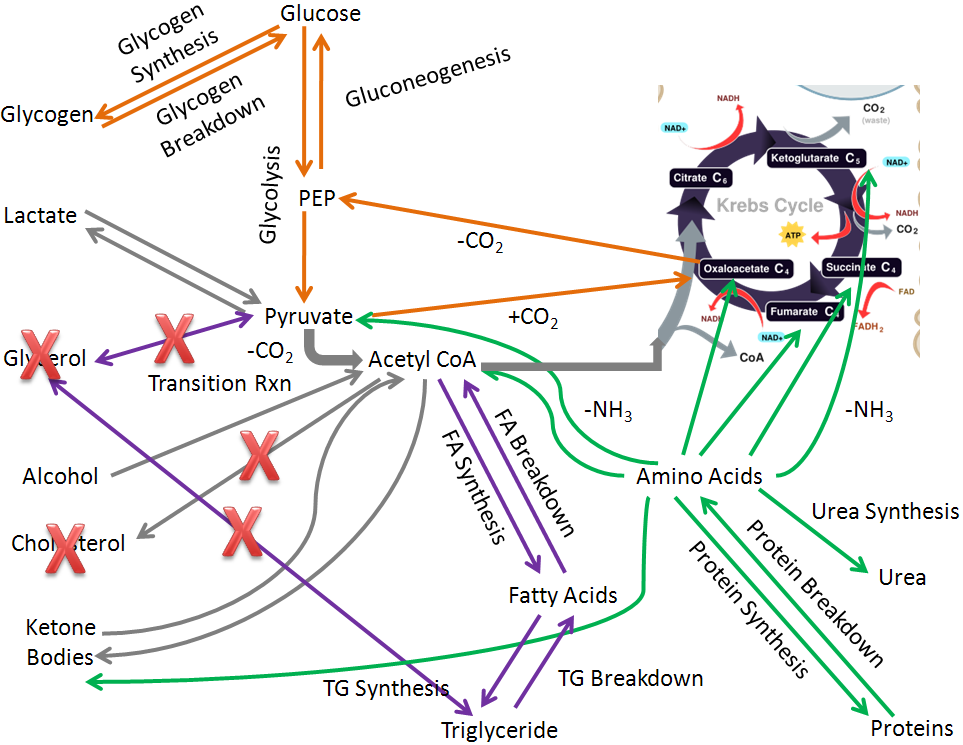
Thus, we are left with the following simplified figure:
Notice that acetyl-CoA is the the central metabolite in integrated metabolism that connects many different pathways. For example, carbohydrates can be broken down to acetyl-CoA that can then be used to synthesize fats and ultimately triglycerides.
References & Links
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
7.2 Liver Macronutrient and Alcohol Metabolism
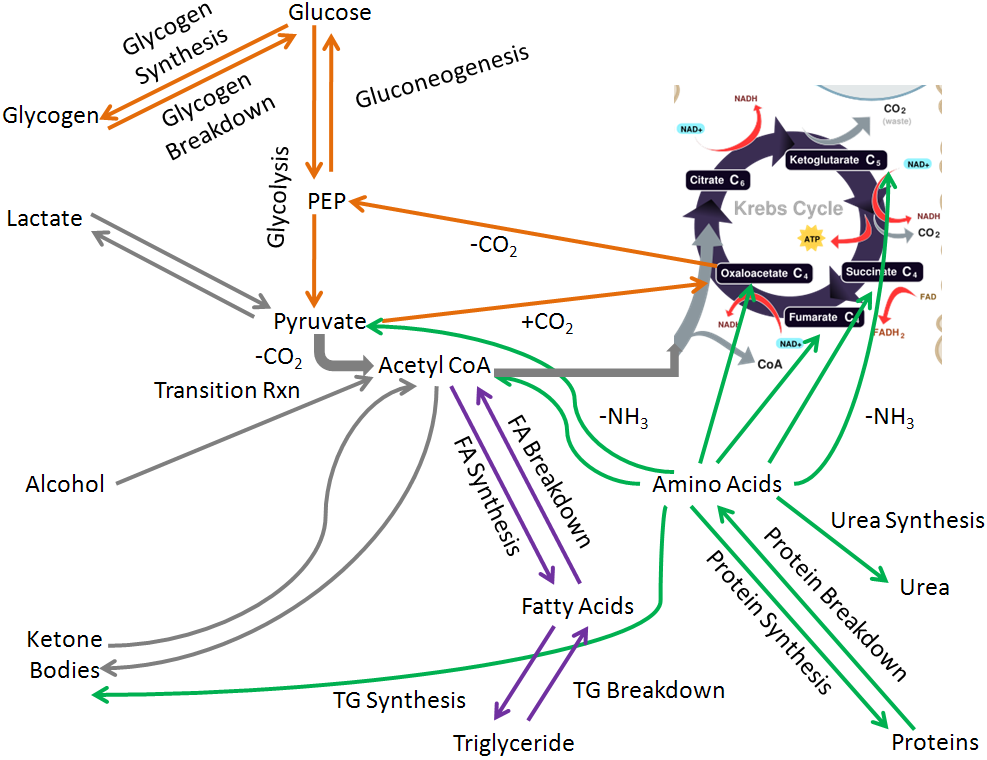
The liver is the organ that has the greatest macronutrient metabolic capability; there are a number of metabolic functions that only the liver performs. However, there are two major macronutrient metabolic processes, lactate synthesis and ketone body breakdown, that the liver will not normally perform, as shown in the figure below.
But aside from those two pathways, the liver performs all the other metabolic pathways that you have learned about that are listed and shown below:
Glycogen synthesis and breakdown
Glycolysis
Gluconeogenesis
Alcohol oxidation
Ketone body synthesis
Fatty acid synthesis and breakdown
Triglyceride synthesis and breakdown
Protein synthesis and breakdown
Urea synthesis
VLDL synthesis
Glucose-6-phosphatase
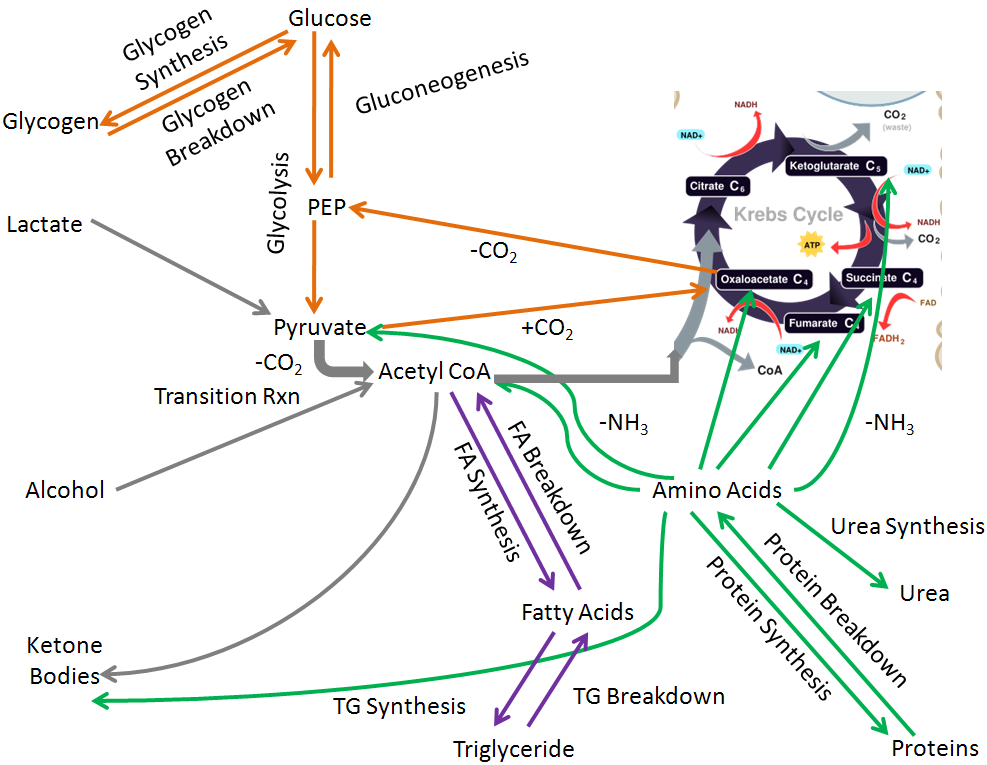
The liver is the only tissue that performs the following functions:
Ketone body synthesis
Urea synthesis
VLDL synthesis
The liver is also the primary, but not exclusive site, of the following functions:
Alcohol oxidation (also occurs in the stomach)
Gluconeogenesis (also occurs in the kidney(s))
Glucose-6-phosphatase activity (also occurs in the kidney(s))
Lactate breakdown (also occurs in muscle)2
Glucose-6-phosphatase is important because it removes the phosphate from glucose-6-phosphate so that glucose can be released into circulation. Kidneys can perform gluconeogenesis and has glucose-6-phosphatase. However, it is estimated that 90% of glucose formed from gluconeogenesis is produced by the liver; the remaining 10% is produced by the kidney(s). It is also important to note that the muscle does not have this enzyme, so it cannot release glucose into circulation3.
References & Links
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
- Phypers B, Pierce JMT. (2006) Lactate physiology in health and disease. Continuing Education in Anaesthesia Critical Care & Pain, 6(3).
- Stipanuk MH. (2006) Biochemical, physiological, & molecular aspects of human nutrition. St. Louis, MO: Saunders Elsevier.
7.3 Extrahepatic Macronutrient Metabolism
Because the liver is so important in metabolism, the term extrahepatic has been defined to mean “located or occurring outside of the liver1”. We are next going to consider extrahepatic tissue metabolism.

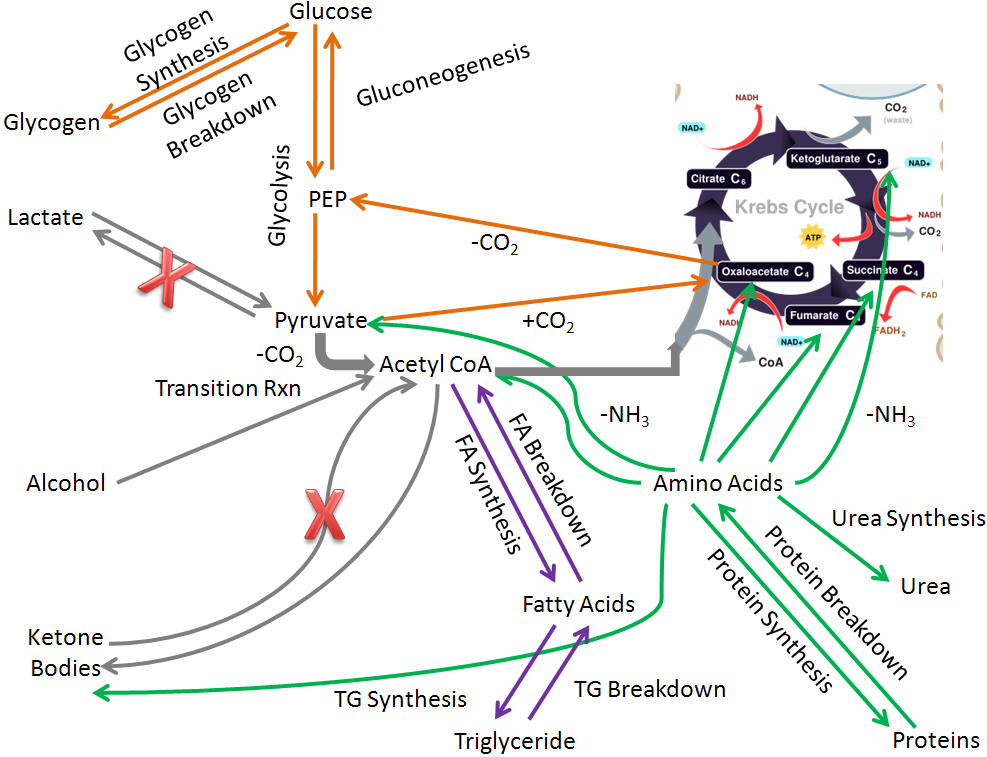
To start considering the metabolic capabilities of the extrahepatic tissues, we start by removing pathways that only or mostly occur in the liver:
Alcohol oxidation
Gluconeogenesis
Ketone body synthesis
Urea synthesis
Lactate breakdown
Glucose-6-phosphatase
These metabolic processes are crossed off in the figure below.
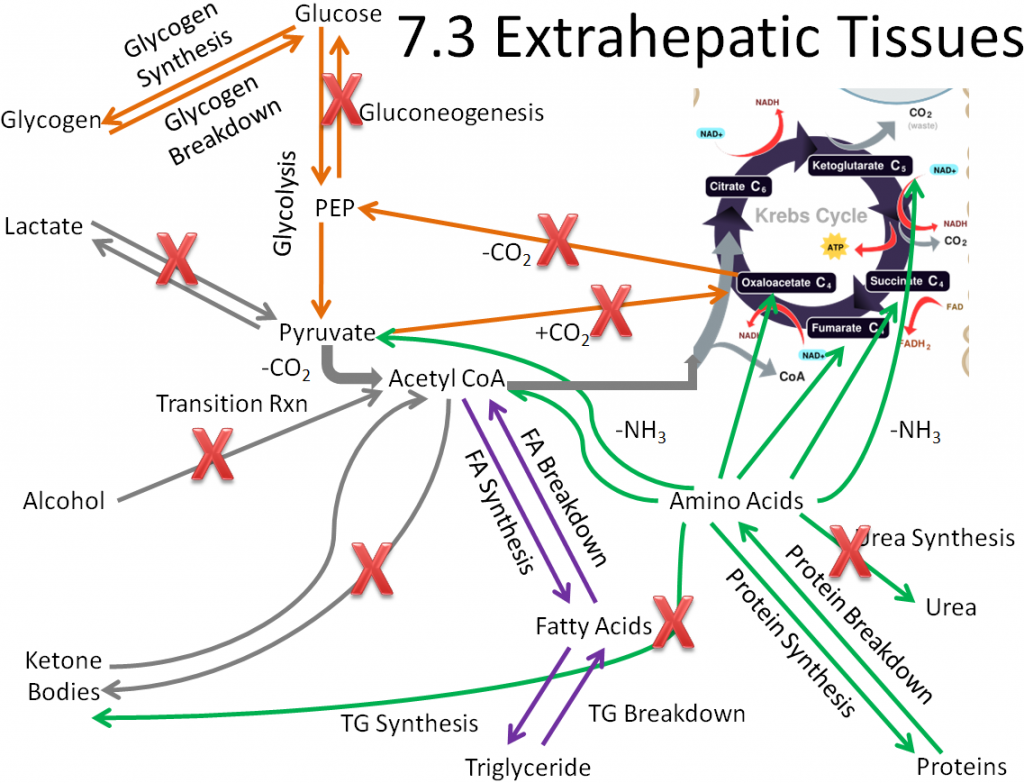
We are left with metabolic capabilities that are listed and shown below.
Glycogen synthesis and breakdown
Glycolysis
Fatty acid synthesis and breakdown
Triglyceride synthesis and breakdown
Protein synthesis and breakdown
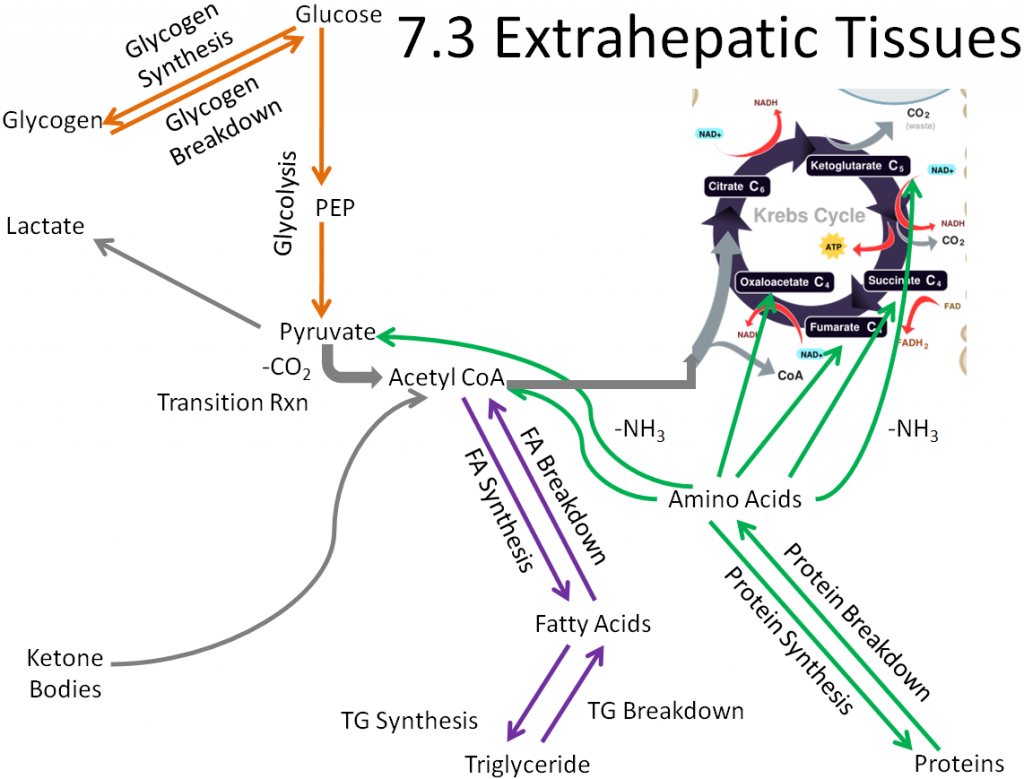
We will use this figure as the base for metabolic capabilities of the different extrahepatic tissues to compare what pathways other tissues can perform versus all the pathways performed by extrahepatic tissues.
In an effort to keep this simple, we are going to focus on four extrahepatic tissues in the following subsections:
7.31 Muscle Macronutrient Metabolism
7.32 Adipose Macronutrient Metabolism
7.33 Brain Macronutrient Metabolism
7.34 Red Blood Cell Macronutrient Metabolism
References & Links
- http://www.cancer.gov/dictionary/?CdrID=44498
- http://commons.wikimedia.org/wiki/File:Liver.svg
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
7.31 Muscle Macronutrient Metabolism
Compared to extrahepatic tissues as a whole, in the muscle the following pathways are not performed or are not important:
Fatty acid synthesis
Ketone body breakdown
These pathways are crossed out in the figure below.
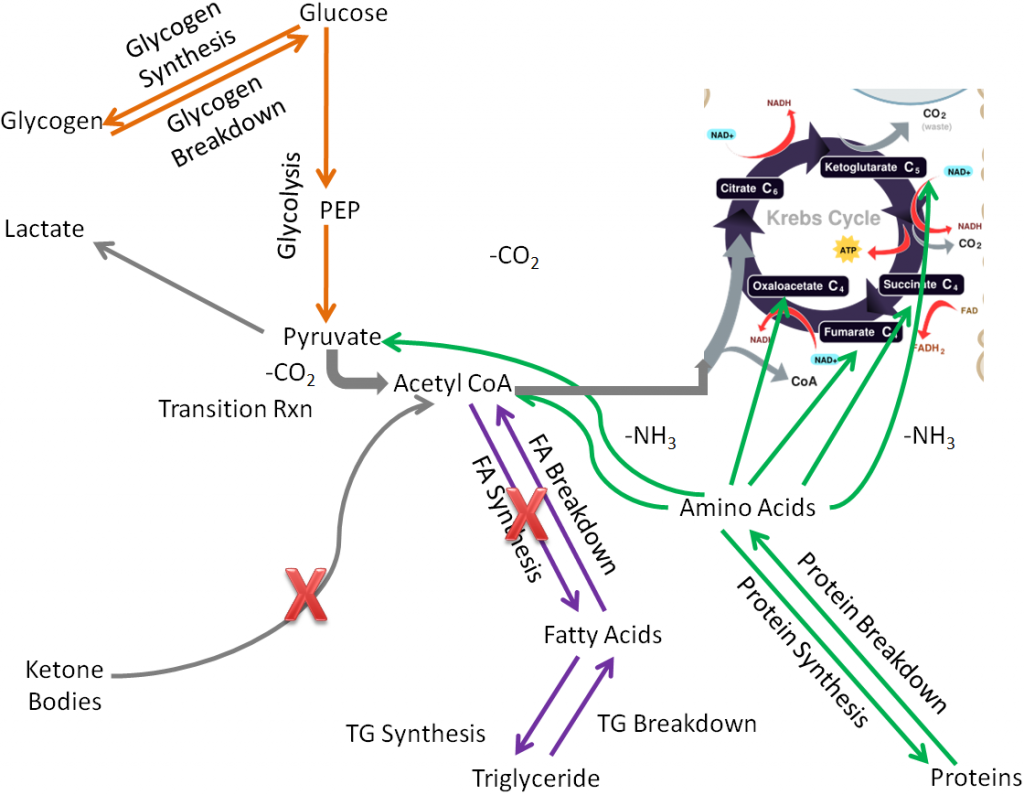
Removing those pathways, the following metabolic pathways make up the muscle metabolic capability:
Glycogen synthesis and breakdown
Glycolysis
Protein synthesis and breakdown
Triglyceride synthesis and breakdown
Fatty acid breakdown
Lactate synthesis
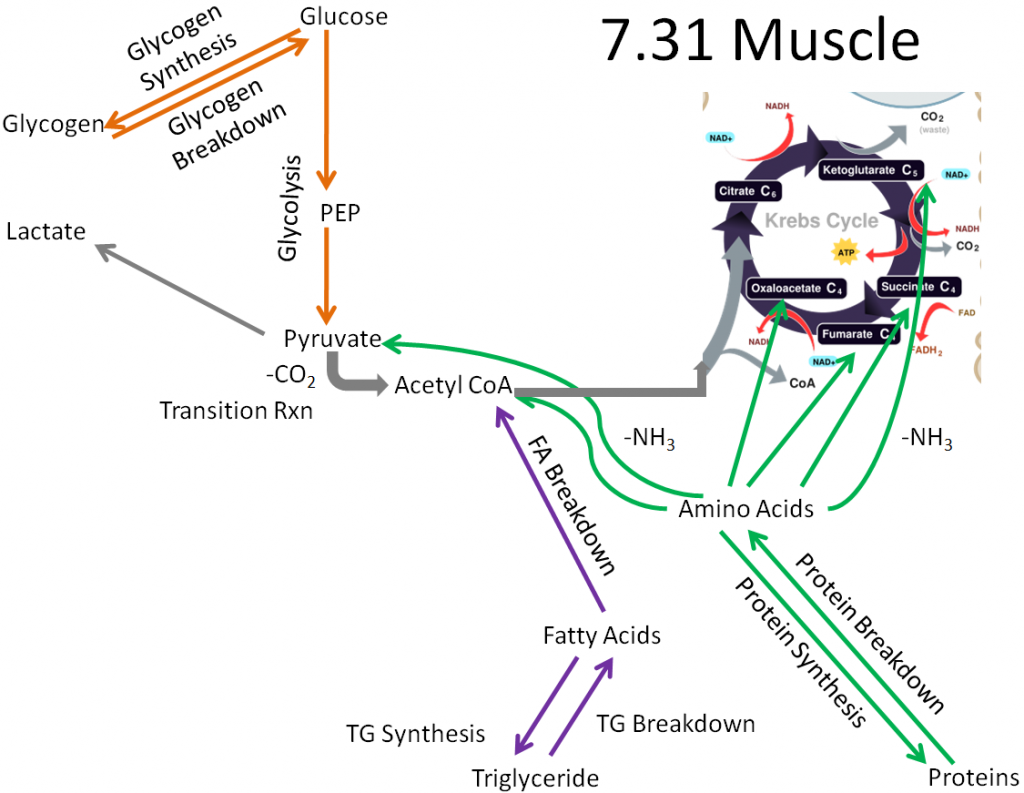
Muscle is a major extrahepatic metabolic tissue. It is the only extrahepatic tissue with significant glycogen stores. However, unlike the liver, the muscle cannot secrete glucose after it is taken up (no glucose-6-phosphatase). Thus, you can think of the muscle as being selfish with glucose. It either uses it for itself initially or stores it for its later use.
References & Links
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
7.32 Adipose Macronutrient Metabolism
It probably does not surprise you that the major function of the adipose is to store energy as triglycerides. Compared to extrahepatic tissues as a whole, in the adipose the following pathways are not performed or are not important:
Glycogen synthesis and breakdown
Lactate synthesis
Ketone body breakdown
Fatty acid breakdown
Protein synthesis and breakdown
Citric acid cycle (not much since it is not an active tissue needing energy)
These pathways are crossed out in the figure below.
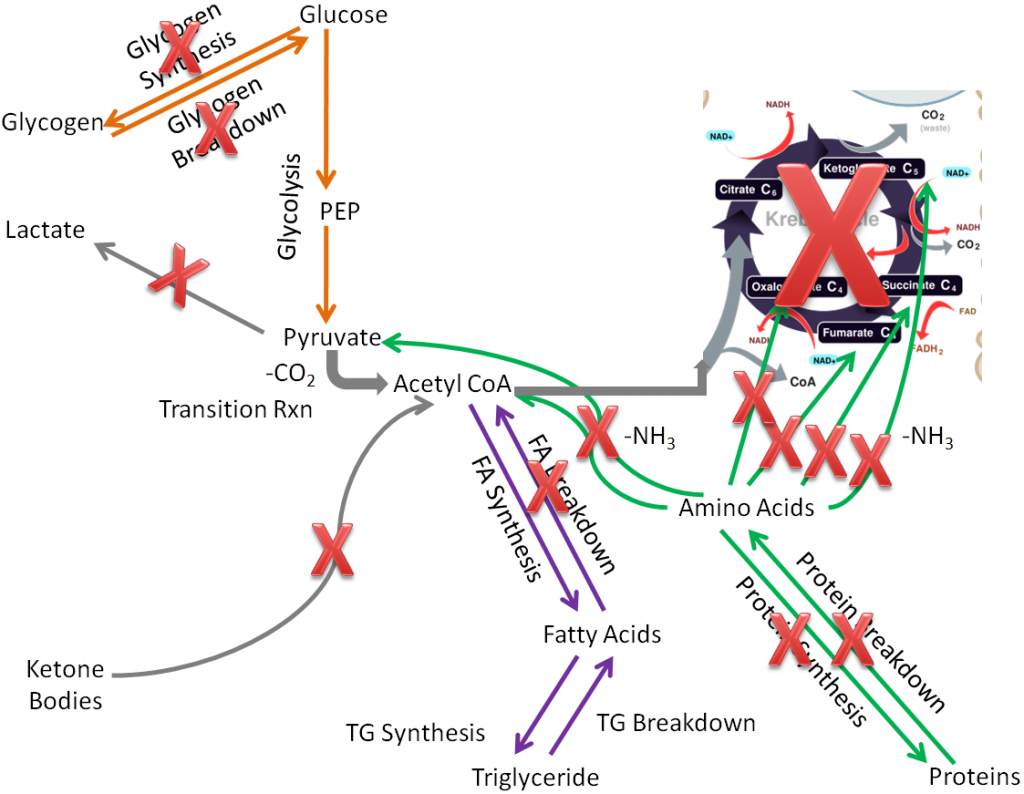
Removing those pathways, we are left with metabolic capabilities listed below and depicted in the following figure:
Glycolysis
Fatty acid synthesis
Triglyceride synthesis and breakdown
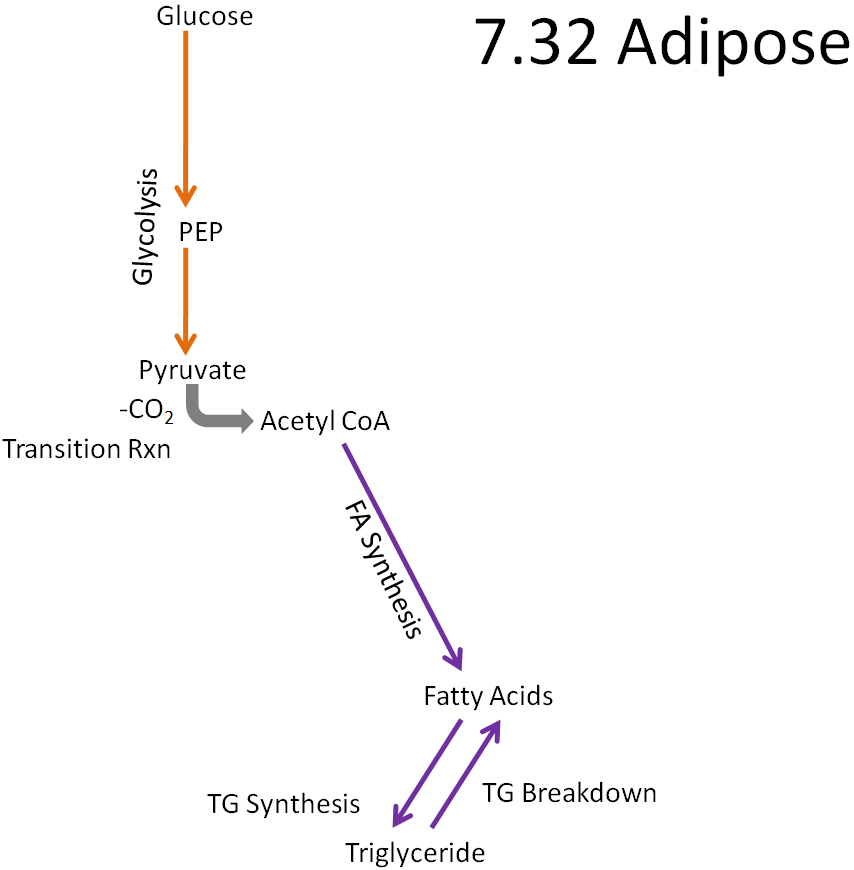
Fatty acid synthesis only occurs in the adipose and liver. In the adipose, fatty acids are synthesized and most will be esterified into triglycerides to be stored. In the liver, some fatty acids will be esterified into triglycerides to be stored, but most triglycerides will be incorporated into VLDL so that they can be used or stored by other tissues.
References & Links
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
7.33 Brain Macronutrient Metabolism
Fatty acid breakdown does not occur to any great extent in the brain because of the low activity of an enzyme in the beta-oxidation pathway limits the pathway’s activity1. Compared to the extrahepatic tissues as a whole, in the brain the following pathways are not performed or are not important:
Glycogen synthesis and breakdown
Lactate synthesis
Fatty acid synthesis and breakdown
Triglyceride synthesis and breakdown
Protein synthesis and breakdown
These pathways are crossed out on the figure below.
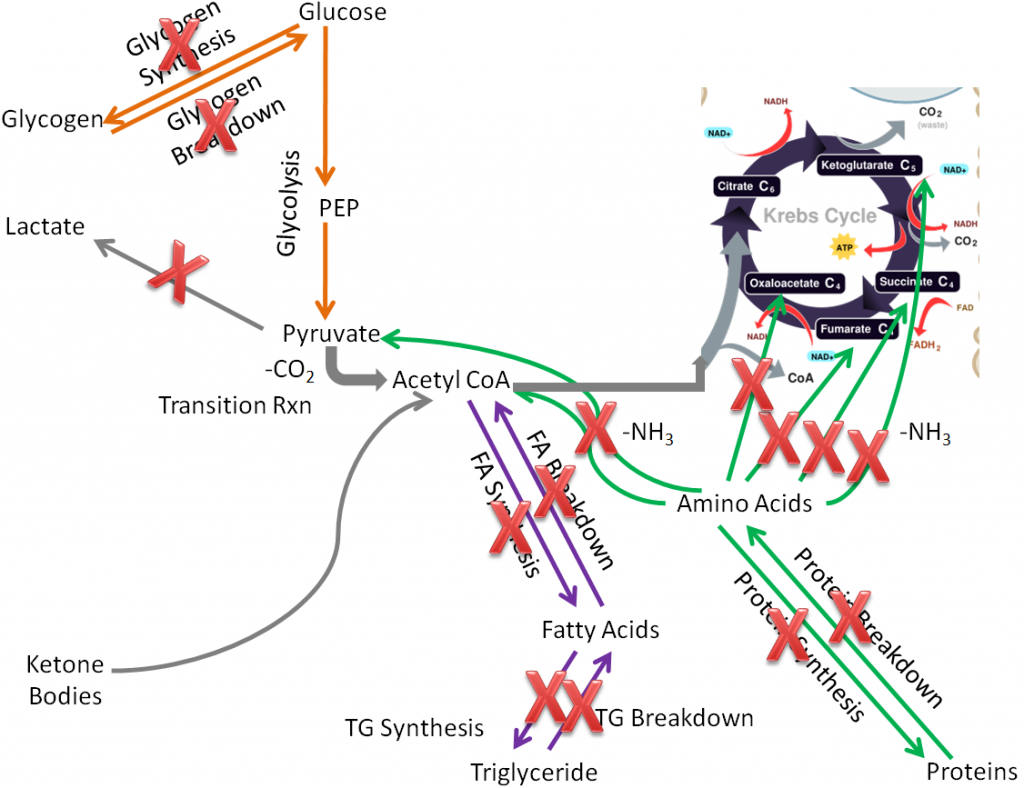
Fatty acid breakdown does not occur to any great extent in the brain because low activity of an enzyme in the beta-oxidation pathway limits the activity of this pathway2.
By removing those pathways the only pathways left are:
Glycolysis
Ketone body breakdown
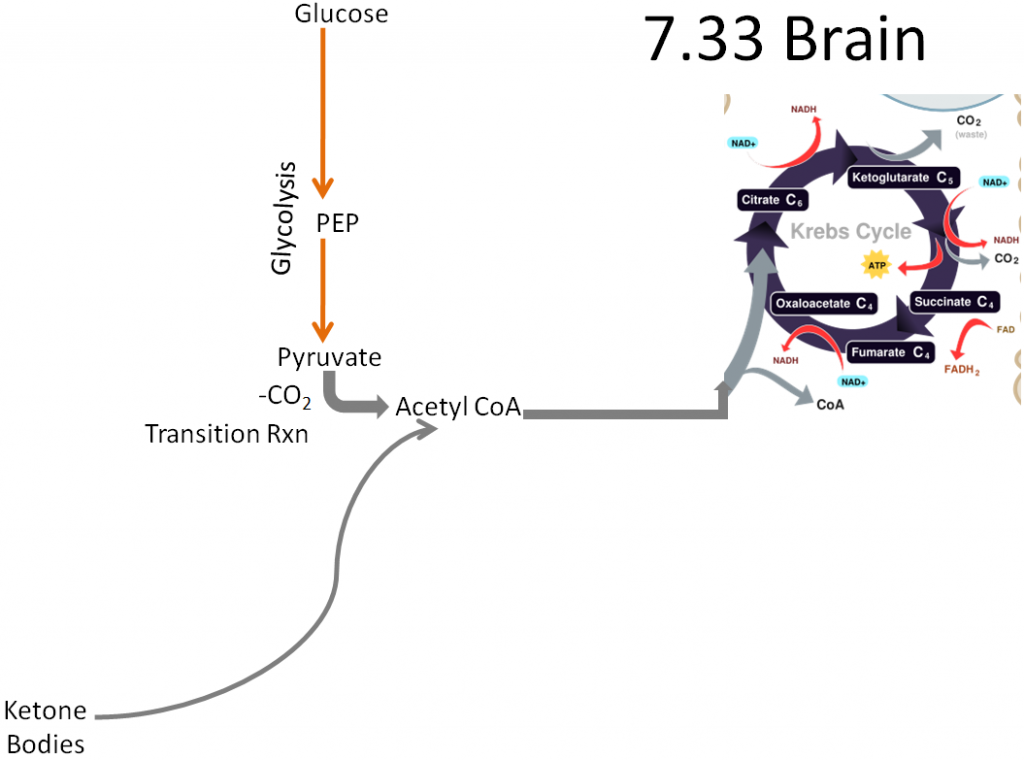
Thus, due to its limited metabolic capabilities, the brain needs to receive either glucose or ketone bodies to use as an energy source.
References & Links
- Yang SY, He XY, Schulz H (1987) Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J BIol Chem 262 (27): 13027-13032.
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
7.34 Red Blood Cell Macronutrient Metabolism
Red blood cells are the most limited of the extrahepatic tissues because they do not contain a nucleus or other cell organelles, most notably mitochondria.
As a result, compared to the extrahepatic tissues, in red blood cells the following pathways are not performed or are not important:
Glycogen synthesis and breakdown
Lactate breakdown
Fatty acid synthesis and breakdown
Triglyceride synthesis and breakdown
Protein synthesis and breakdown
Ketone body breakdown
These pathways are crossed off in the figure below.
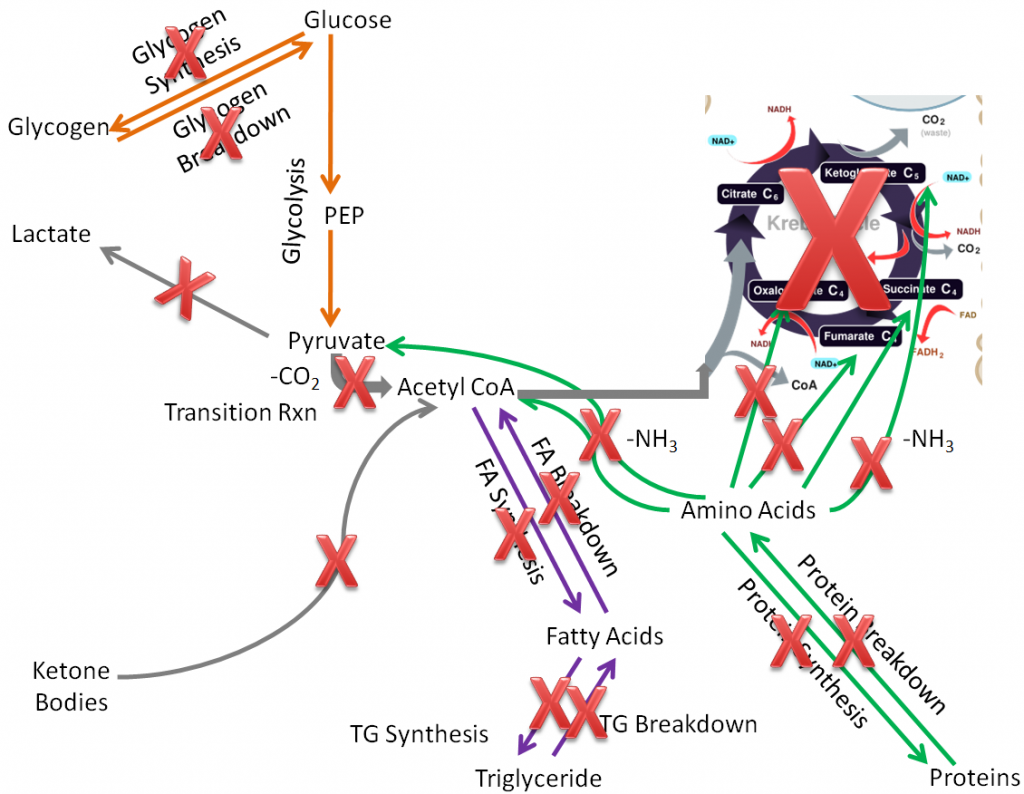
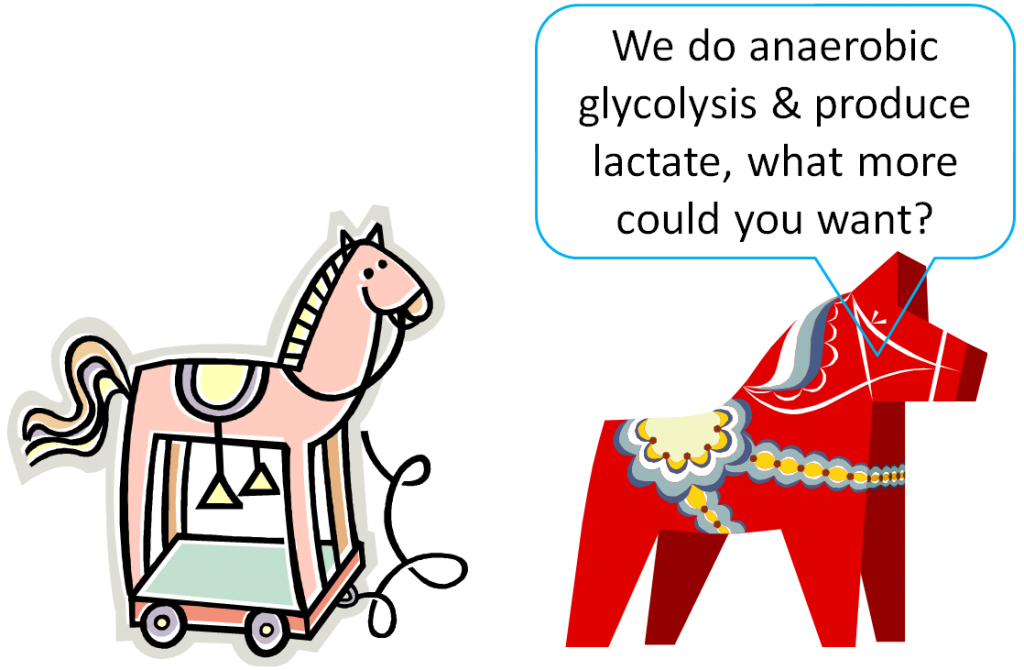
If all those pathways are removed, only glycolysis is left, where pyruvate is converted to lactate.
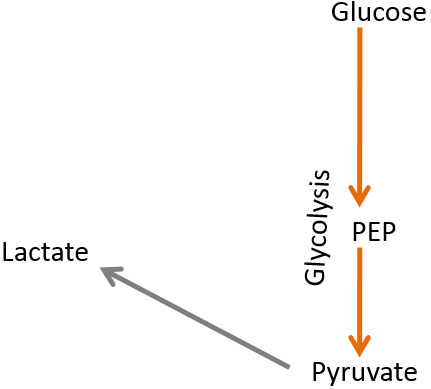
Thus, red blood cells are one-trick ponies, only being able to perform glycolysis and produce lactate.
References & Links
- http://en.wikipedia.org/wiki/Mitochondrion
- http://en.wikipedia.org/wiki/File:CellRespiration.svg
7.4 Metabolic Conditions
You have learned about the pathways and the tissue metabolic capabilities, so now we’re going to apply that knowledge to three conditions: fasting, the Atkins diet, and the Ornish/Pritikin diet, as ways to illustrate how you can use this knowledge. In fasting, we’re going to be considering what is happening metabolically during a prolonged period without food. This is a catabolic condition. The Atkins diet is a carbohydrate-restricted diet, so we are going to consider what happens metabolically when someone is eating a diet that essentially only contains protein and lipids over an extended period of time. This is an anabolic condition. Finally the Ornish/Pritikin diet is a very low fat diet, so we’re going to consider what happens metabolically when someone is eating a diet that is essentially only carbohydrates and protein over an extended period of time. This is an anabolic condition. For each of these conditions, we’re going to consider what is happening in the liver, muscle, adipose, and brain.
Now that you should have an understanding of the glycemic response and macronutrient metabolism, you should be able to understand the broader effects of insulin and glucagon that are summarized in the following tables. Knowing what hormone is elevated in the different conditions helps you to understand the metabolism that occurs in different conditions.
Table 7.41 Insulin’s effects on targets in tissues1,2
| Effect | Tissue | Target |
| ↑ Glucose Uptake | Muscle, Adipose | ↑ GLUT4 |
| ↑ Glucose Uptake | Liver | ↑ Glucokinase |
| ↑ Glycogen Synthesis | Liver, Muscle | ↑ Glycogen Synthase |
| ↓ Glycogen Breakdown | Liver, Muscle | ↓ Glycogen Phosphorylase |
| ↑ Glycolysis,
↑ Transition Reaction |
Liver, Muscle | ↑ Phosphofructokinase-1
↑ Pyruvate Dehydrogenase Complex |
| ↑ Fatty Acid Synthesis | Liver | ↑ Fatty Acid Synthase |
| ↑ Triglyceride Synthesis | Adipose | ↑ Lipoprotein Lipase |
Table 7.42 Glucagon’s effects on targets in tissues2
| Effect | Tissue | Target |
| ↑ Glycogen Breakdown | Liver | ↑ Glycogen Phosphorylase |
| ↓ Glycogen Synthesis | Liver | ↓ Glycogen Synthase |
| ↑ Gluconeogenesis | Liver | Multiple Enzymes |
| ↓ Glycolysis | Liver | ↓ Phosphofructokinase-1 |
| ↑ Ketone Body Synthesis | Liver | ↑ Acetyl-CoA Carboxylase |
| ↑ Triglyceride Breakdown | Adipose | ↑ Hormone-Sensitive Lipase |
Subsections:
7.41 Fasting
7.42 Atkins Diet
7.43 Ornish/Pritikin Diet
References & Links
- Gropper SS, Smith JL, Groff JL. (2008) Advanced nutrition and human metabolism. Belmont, CA: Wadsworth Publishing.
- http://jpkc.gmu.cn/swhx/book/shyl/23.pdf
7.41 Fasting
In this condition a person has been fasting for an extended period of time (18 hours or longer). As a result, the person is in a catabolic state with low blood glucose levels, which leads the pancreas to secrete glucagon.
The liver will break down glycogen to secrete glucose for other tissues to use until its stores are exhausted. Amino acids and lactate from muscle will be used for gluconeogenesis to synthesize glucose that will also be secreted. Glycolysis will not be occurring to any great extent to spare glucose for use by other tissues. From the breakdown of amino acids, there will be an increase in the synthesis and secretion of urea from the liver to safely rid the body of ammonia from the amino acids. Fatty acids that are received from the adipose will be broken down to acetyl-CoA and used to synthesize ketone bodies that are secreted for tissues, such as the brain, that cannot directly use fatty acids as a fuel.
The muscle will break down glycogen to glucose until glycogen stores are exhausted, and receive glucose from the liver that enters glycolysis, forming pyruvate. Glucose will be used for anaerobic (lactate) and aerobic (pyruvate) respiration. Pyruvate will enter the transition reaction to form acetyl-CoA. The acetyl-CoA will then enter the citric acid cycle, and NADH and FADH2 produced will enter the electron transport chain to generate ATP. Once there isn’t enough glucose for the muscle to use, fatty acids taken up from the adipose and from breakdown of muscle triglyceride stores will be broken down to acetyl-CoA. The acetyl-CoA will then enter the citric acid cycle, and NADH and FADH2 produced will enter the electron transport chain to generate ATP. Amino acids from protein breakdown and lactate (Cori Cycle) will be secreted to be used by the liver for gluconeogenesis.
The adipose tissue will break down triglycerides to fatty acids and release these for use by the muscle and the liver. It will not be taking up anything.
No References
7.42 Atkins Diet
In this condition, assume a person just started into phase I of the Atkins Diet and he/she has just consumed a meal of all protein and fat with no carbohydrates. As a result, this person is in an anabolic state, but blood glucose levels are low, meaning the pancreas will secrete glucagon.
Liver glycogen stores will be broken down to secrete glucose for other tissues. Glycolysis will not be occurring to any great extent, in order to spare glucose for other tissues. Using amino acids from digestion and lactate from muscle, gluconeogenesis will synthesize glucose that will also be secreted. From the breakdown of amino acids, there will be an increase in the synthesis and secretion of urea from the liver to safely rid the body of ammonia from the amino acids. Amino acids will also be used for protein synthesis. Some triglycerides from chylomicron remnants taken up will be broken down to fatty acids. These will then be broken down to acetyl-CoA and used to synthesize ketone bodies that are secreted for tissues, such as the brain, that cannot directly use fatty acids as a fuel. Other triglycerides will be packaged into VLDL and secreted from the liver.
The muscle is going to break down glycogen to glucose, and receive glucose from the liver that enters glycolysis, forming pyruvate. Glucose will be used for anaerobic (lactate) and aerobic (pyruvate) respiration. After glycogen is used up, most glucose will be used for anaerobic respiration to spare glucose. In aerobic respiration, pyruvate will enter the transition reaction to form acetyl-CoA. The acetyl-CoA will then enter the citric acid cycle, and NADH and FADH2 produced will enter the electron transport chain to generate ATP. Once there is not enough glucose for the muscle to use, fatty acids will be cleaved from and taken up from chylomicrons, chylomicron remnants, VLDL, IDL, and LDL and broken down to acetyl-CoA. The acetyl-CoA will then enter the citric acid cycle, and NADH and FADH2 produced will enter the electron transport chain to generate ATP. Amino acids taken up will be used for protein synthesis, and lactate will be secreted for the liver to use for gluconeogenesis (Cori cycle).
In the adipose, fatty acids that are cleaved from chylomicrons, chylomicron remnants, VLDL, IDL, and LDL are also going to be taken up. These fatty acids will be used to synthesize triglycerides for storage. With glucagon levels high in this condition, hormone-sensitive lipase would be active. However, since this is an anabolic state, the net effect would be uptake of fatty acids after cleavage by lipoprotein lipase. The adipose won’t be secreting anything under this condition.
No References
7.43 Ornish/Pritikin Diet
In this condition, assume a person is on the Ornish/Pritikin diet and just consumed a meal containing carbohydrates, with minimal but adequate amount of protein and no fat. As a result, this person is in an anabolic state with high blood glucose levels, meaning the pancreas will secrete insulin.
The liver will take up glucose and synthesize glycogen until its stores are filled. After these stores are full, glucose can be broken down through glycolysis to pyruvate, then form acetyl-CoA in the transition reaction. Because we are in the fed or anabolic state, acetyl-CoA will be used for fatty acid synthesis, and the fatty acids will be used for triglyceride synthesis. However, evidence suggests that this de novo lipogenesis pathway does not occur to any great extent in humans1. These triglycerides will be packaged into VLDL and secreted from the liver. Amino acids will also be taken up and used for protein synthesis as needed. Because there is plenty of glucose, gluconeogenesis and ketone body synthesis will not be operating to any great extent.
The muscle will take up glucose and synthesize glycogen until those stores are filled. Some glucose will go through glycolysis to produce pyruvate, then form acetyl-CoA in the transition reaction. The acetyl-CoA will enter the citric acid cycle, and NADH and FADH2 produced will enter the electron transport chain to generate ATP. Fatty acids that are cleaved from VLDL, IDL, and LDL are also going to be taken up. These fatty acids will be used to synthesize triglycerides for storage. Whatever amino acids are taken up will be used for protein synthesis. The muscle will not be secreting anything in this condition.
The adipose is going to take up glucose that will enter glycolysis, where pyruvate will be produced, then acetyl-CoA will be produced in the transition reaction. Because we are in the fed or anabolic state, the acetyl-CoA will be used for fatty acid synthesis, and the fatty acids will be used for triglyceride synthesis. However, evidence suggests that de novo lipogenesis does not occur to any great extent in humans1. Fatty acids that are cleaved from VLDL, IDL, and LDL are going to be taken up and primarily used to synthesize triglycerides for storage. The adipose won’t be secreting anything under this condition.
The brain will have plenty of glucose available for its use, so it is not going to have to use ketone bodies like it would during fasting and during prolonged Atkins diet consumption.
References & Links
- McDevitt RM, Bott SJ, Harding M, Coward WA, Bluck LJ, et al. (2001) De novo lipogenesis during controlled overfeeding with sucrose or glucose in lean and obese women. Am J Clin Nutr 74(6): 737-746.

