IX. Proteins
This chapter provides an introduction and discussion of proteins and amino acids that are important in the nutrition of food-producing animals.
New Terms
Amino acid
Dipeptide
Essential amino acid
Nonessential amino acid
Peptide bond
Polypeptide
Protein
Chapter Objectives
- To describe the chemical structure of proteins and amino acids
- To discuss the different classification of protein and amino acids
- To discuss essential and nonessential amino acids
Proteins
What Are Proteins?
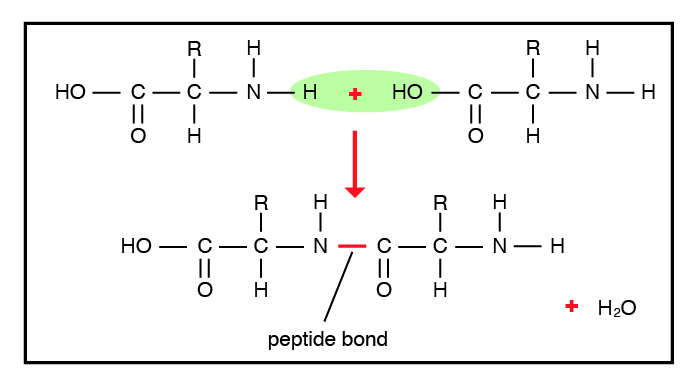
The word proteins was coined by a Dutch chemist G. J. Mulder and originated from the Greek word “proteios”, meaning first or most important. Proteins are organic compounds made up of different building blocks (basic units) called amino acids joined together by peptide bonds (Figure 9.1). A dipeptide contains one peptide bond and two amino acids, whereas a tripeptide contains three amino acids and two peptide bonds. A peptide with more than ten amino acids is called a polypeptide. Proteins are essentially large polypeptides. The structure of a protein is determined first by the sequence of individual amino acids it has in the polypeptide chain. This is also called the primary structure of the protein.
Protein: Functions
- Body proteins (e.g., muscle, hair, hooves, skin)
- Blood proteins (e.g., albumin, globulin)
- Tissue proteins (e.g., collagen, keratin)
- Enzymes and hormones
- Immune system antibodies and other peptide growth factors
Protein Functions: Proteins are vital for life and are the major structural components of animal tissues (e.g., skin, muscles, wool, feather, tendons, eggs). In addition, proteins are also involved in biochemical (e.g., enzymes), immunological (e.g., immunoglobulins), transportational (e.g., lipoproteins), and other regulatory (e.g., hormones) activities. Proteins can also provide energy when needed.
Many of the structures in animal tissue (e.g., muscle) and metabolic reactions (e.g., enzymes, hormones) are catalyzed by proteins. Therefore, protein synthesis is essential for maintaining life process. Provision of adequate dietary protein and amino acids are essential for maintaining growth, health, and productivity in food-producing animals. Intestinal microflora can synthesize proteins from nonprotein sources in ruminant animals.
Protein requirements vary with life stages and are high during phases of fast growth in young animals and during pregnancy and lactation. Like other macronutrients, proteins contain carbon, oxygen, and hydrogen. In addition, proteins also contain nitrogen and sulfur (in some amino acids). It is the nitrogen that makes proteins very unique in animal nutrition with respect to its digestibility, metabolism, and disposal within the animal body.
Classification of Proteins
Proteins can be classified based on their shape; solubility in water, salt, acid, base, or alcohol; or according to the nature of the prosthetic group.
Classification Based on Solubility and Prosthetic Group
-
- Globular proteins: soluble in water or dilute acids, bases, or alcohol
- Albumin (water soluble; present as albumen in egg white; in blood circulation, it performs various functions [e.g., as a carrier of lipids])
- Globulin (soluble in dilute neutral solutions; functions as part of the immune system in body defense [e.g., immunoglobulins]
- Fibrous proteins: insoluble in water and are resistant to digestive enzymes
- Keratins (e.g. wool, hair, feather, hooves, horn)
- Collagen (can be converted to gelatin when heated; present in bone, teeth, tendons, and soft connective tissue)
- Conjugated proteins: contain other nonprotein compounds in structure. Some examples follow:
- Lipoproteins (lipid-carrying protein)
- Hemoprotein (proteins with heme units)
- Glycoproteins (proteins with sugar)
- Nucleoprotein (proteins bound to nucleic acid
- Globular proteins: soluble in water or dilute acids, bases, or alcohol
These proteins have limited nutritional value but are important in biochemical, structural, and other metabolic functions. For example, feather meal is high in protein (keratin) but very low in digestibility and is of limited use in animal nutrition as a feed ingredient. Amino acids in the polypeptide chain in feather meal form disulfide bonds (-S-S-), which twist the polypeptide chain into a specific coiled structure such as helix or sheet. This is called a secondary structure. These bonds account for the tough physical properties of hooves and horns and their low digestibility.
The disruption of secondary structure by heat treatment causes denaturation of the proteins (e.g., egg white coagulation during cooking). Certain antinutritional factors in feed (e.g., trypsin inhibitor in soybean meal) are proteins. Heat processing denatures trypsin inhibitor in soybean meal and can enhance digestibility.
Amino Acids: Amino acids are the building blocks of proteins. There are more than 300 different amino acids known to exist in nature. Out of these, about 20 amino acids are important constituents of animal proteins and are associated with muscles, connective tissues, skin, feathers, horns, blood, enzymes, and hormones. There about 10 amino acids that should be present in the diet of animals because animal tissues cannot synthesize them or cannot make the adequate amount needed for metabolic functions; these are called essential amino acids. A few other amino acids such as citrulline and ornithine do not occur in animal tissues but are involved in cellular metabolic functions.
Animals cannot synthesize them or cannot make the adequate amount needed.
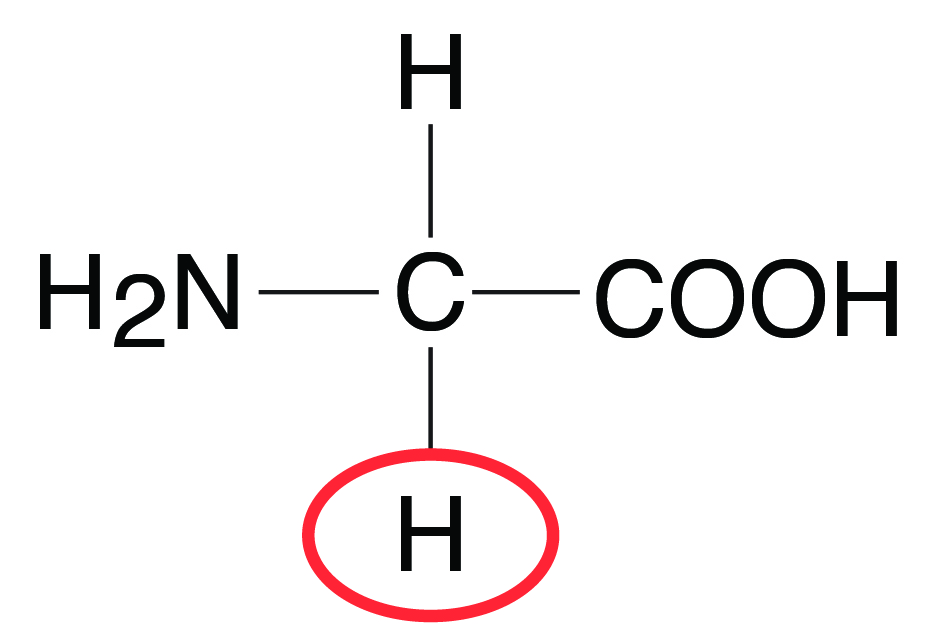
All amino acids by definition contain at least one amino group (-NH2) and one carboxyl group (–COOH) on the C atom adjacent to the carboxyl group (Figure 9.2). An exception to this is proline (imino acid), which is lacking a free amino group. The general structure of an amino acid is shown below by the amino acid glycine, the simplest of the amino acids (Figure 9.2). The R group (shown in the red circle) in amino acids varies for different amino acids. The R group is the remainder of the molecule or any other group attached to the C atom. In the case of glycine, it is an H group. The amino group (NH2) provides basic properties to the amino acid, and the carboxyl (COOH) group provides acidic properties. Amino acids important in animal nutrition are alpha (α) amino acids, which are carboxylic acids with an amino group on the α-carbon (or the first carbon attached to a functional group). A list of amino acids important in animal nutrition, their essentiality and classification are shown in Table 9.1.
Amino acids can exist in two isomeric forms, the D- and L-isomers. The D- and L-amino acids differ in their configuration of groups around the asymmetric α-carbon. Only L-amino acids are used in protein synthesis, except methionine, where both D- and L-amino acids can be used by the animal. DL methionine is commonly used as an amino acid supplement in animal feeds.
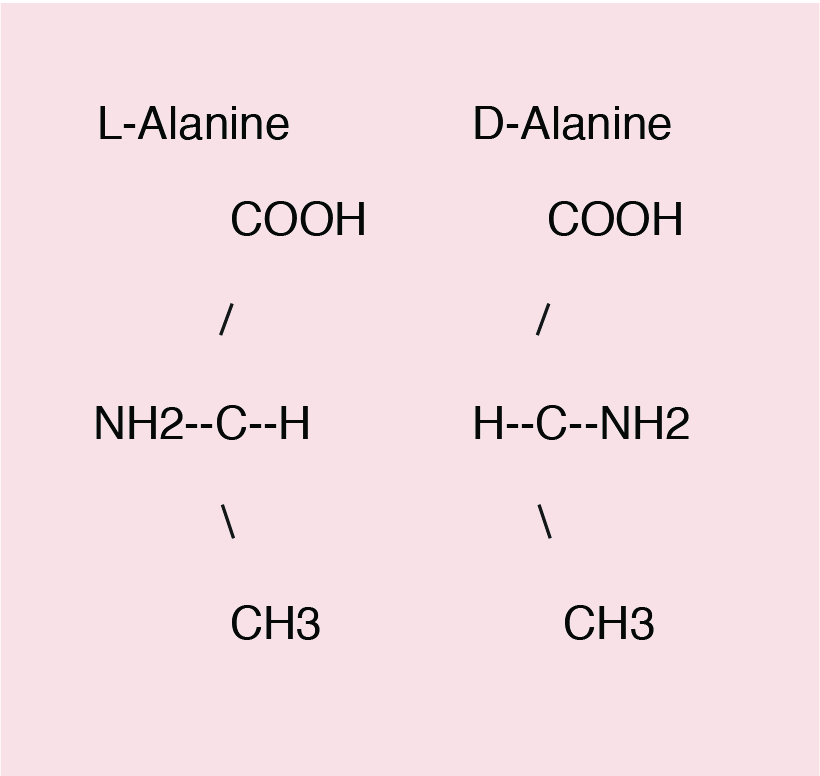 All amino acids except glycine contain an asymmetric α-carbon (with four different chemical groups attached to it). Compounds with asymmetric carbons can exist as isomers.
All amino acids except glycine contain an asymmetric α-carbon (with four different chemical groups attached to it). Compounds with asymmetric carbons can exist as isomers.
Essential Amino Acids: Animal body can synthesize some amino acids in sufficient amounts. However, animals cannot synthesize some amino acids, or not in the amount that is needed for body requirements. Such amino acids need to be provided through diet in monogastric animals; these are called essential (indispensable) amino acids. Pigs, dogs, and humans need a total of 10, and chickens and cats need a total of 11 essential amino acids. A list of essential amino acids needed by monogastric animals is shown below.
It should be borne in mind that other nonessential amino acids are also physiologically important for metabolic functions in the body and are made from other precursors available through diet (e.g., carbohydrates, nonprotein nitrogenous substances).
The need for essential amino acids varies in animals. For example, horses need essential amino acids, whereas ruminant animals (e.g., cattle, sheep, goats) generally do not have a requirement of essential amino acids as they are synthesized by rumen microbes.
List of Essential Amino Acids and Their Common Abbreviations
- Arginine (Arg)
- Histidine (His)
- Lysine (Lys)
- Isoleucine (Ile)
- Leucine (Leu)
- Methionine (Met)
- Phenylalanine (Phe)
- Threonine (Thr)
- Tryptophan (Try)
- Valine (Val)
In addition to these 10 essential amino acids, cats and chickens need the following extra amino acids.
Cats need taurine (Tau), and chickens need glycine (Gly).
| Amino acid | Essentiality | Classification |
| Ariginine (Arg) | E | Basic |
| Histidine (His) | E | Basic |
| Lysine (Lys) | E | Basic |
| Aspartic acid (Asp) | NE | Acidic |
| Glutamic acid (Glu) | NE | Acidic |
| Alanine (Ala) | NE | Aliphatic-Straight chain |
| Glycine (Gly) | E (chickens) | Neutral- Aliphatic-Straight chain |
| Isoleucine (Ilu) | E | Branched chain |
| Leucine (Leu) | E | Branched chain |
| Valine (Val) | E | Branched chain |
| Serine (Ser) | NE | Hydroxy |
| Threonine (Thr) | E | Branched chain |
| Cysteine (Cys) | NE | Sulfur-containing |
| Methionine (Met) | E | Sulfur-containing |
| Phenylalanine (Phe) | E | Aromatic |
| Tryptophan (Try) | E | Aromatic |
| Tyrosine (Tyr) | Ne | Aromatic |
| Hydroxyproline (Hydro) | Ne | Imino acid |
| Proline (Pro) | Ne | Imino acid |
Key Points
- Proteins can be found in structural components of the body and are needed for many metabolic functions.
- The presence of Nitrogen makes protein unique.
- More than 300 amino acids are identified. But only 20 amino acids are used to synthesize all proteins.
- Three features of a typical amino acid include a carbon skeleton, a carboxyl group, and an amino group.
- Acidic amino acids contain more carboxyl groups, and basic amino acids contain more amino groups. Neutral amino acids contain an equal number of carboxyl and amino groups.
- Sulfur-containing amino acids are methionine and cysteine. Among these amino acids, methionine is essential because animals cannot synthesize it. Cysteine is not considered essential because if S is available, the body can make it.
- Aromatic amino acids contain a ring structure.
- Imino acids contain an imino instead of an amino group (e.g., proline).
- Essential amino acids are those that cannot be synthesized by the animal body. There are 10 essential amino acids; cats need taurine, and chickens need glycine.
- Amino acids are joined together by peptide bonds. A long chain of amino acids formed this way is called a polypeptide.
- The primary structure of an amino acid is determined by the sequence of individual amino acids in the polypeptide chain. The amino acids in the polypeptide chain form disulfide bonds and hydrogen bonds, which twist the polypeptide chain into a specific coiled structure, such as a helix or a sheet. This is called a secondary structure. Proteins can be classified based on their shape; solubility in water, salt, acid, base, or alcohol; or according to the nature of prosthetic groups.
Review Questions
- Name the linkage between two amino acids in a protein.
- What are the essential amino acids? Why are they essential?
- Compared to carbohydrates, why are proteins unique?
- Which amino acid is essential to chickens but not humans? How about to cats?
- Name one amino acid from the following groups: acidic, basic, aromatic, and sulfur-containing.
- Differentiate between essential and nonessential amino acids.
- Is proline an amino acid?
- List the 10 essential amino acids for monogastric animals.
- How many peptide bonds are there in a tripeptide?
- Give an example of a globular, fibrous, and conjugated protein.

