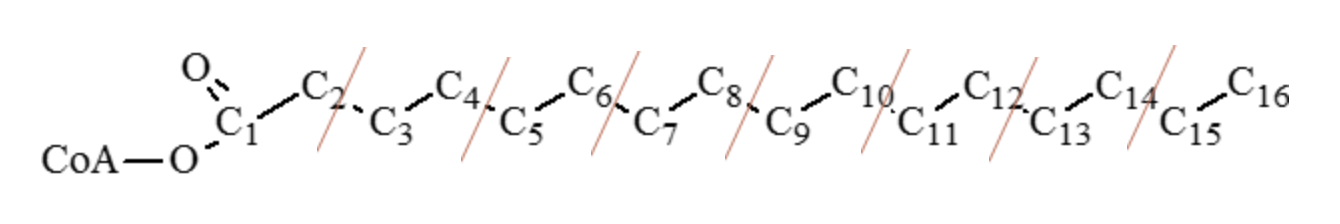VIII. Lipids, Transport, Deposition, and Metabolism
This chapter discusses the transport of digested and absorbed fatty acids in the animal body, depositing in tissues, and synthesis and oxidation of fatty acids for energy production.
Fatty acid synthesis
High-density lipoprotein (HDL)
Ketone bodies
Ketosis
Lipogenesis
Lipoproteins
Low-density lipoprotein (LDL)
Lipoprotein lipase
Malonyl CoA
β-oxidation
Very-low-density lipoprotein (VLDL)
Chapter Objectives
- To discuss the role of lipoproteins in fatty acid transport, storage and metabolism.
- To introduce the transport of digested fatty acids in the body
- To introduce how digested and absorbed fatty acids are mobilized and can serve as energy sources during times of need
Lipid Transport: Blood lipids consist of chylomicrons formed within the intestinal mucosal cells during absorption as well as lipids derived from storage depots, such as liver and adipose tissue. Blood lipids are transported as lipoproteins due to their hydrophobic nature.
Lipoproteins: Lipoproteins consists of an inner core of hydrophobic lipids surrounded by a surface layer of phospholipids, cholesterol, and outer proteins (apolipoprotein).
Main Lipoproteins
- Chylomicron
- VLDL
- LDL
- HDL
Lipoproteins are a lipid + a protein (compound lipid). Because lipids are less dense than water, the density of lipoproteins decreases as the proportion of lipid to protein increases. Lipoproteins have a major role in lipid and cholesterol transport and metabolism.
Lipoproteins are classified based on their density and composition. The main lipoproteins in blood are chylomicrons, very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL).
Chylomicrons are synthesized in the small intestine from dietary fat, and VLDL, LDL, and HDL are synthesized in the liver and small intestine. Chylomicrons enter the liver and are packaged into VLDL. VLDL is involved in the transport of triacylglycerol (TAG) from the liver to extrahepatic tissues. LDL transports cholesterol (so-called bad cholesterol) to tissues and HDL is responsible for “reverse transport” or removal of cholesterol (so-called good cholesterol) from tissues. The terms “bad” and “good” refer to the nature of transport. For example, the “good” cholesterol refers to cholesterol being carried by HDL, which is meant to be routed to the liver for bile formation, or excretion, thus leaving the body and not deposited, like LDL, into blood vessels.
Fatty Acid Metabolism
The liver has a central role in lipid transport and metabolism as it is involved in the synthesis and catabolism of lipoproteins. Pathways of fatty acid degradation and biosynthesis are highly intertwined with pathways of carbohydrate metabolism due to the central role of acetyl CoA in both lipid and carbohydrate metabolism.
Fatty Acid Oxidation
Fatty acids released from the hydrolysis of TAG are transported via blood. Oxidation occurs in the mitochondria of tissues, such as skeletal muscle, cardiac tissue, liver tissue, adipose tissue, and other tissues by converting them to acetyl CoA, which is then catabolized in the citric acid cycle.
Before oxidation begins, fatty acids are activated (they react with CoA) to form fatty acyl CoA (ATP is needed for this step). Activated fatty acids are transported across the mitochondrial membrane (carnitine serves as a carrier). In β-oxidation, two carbons at a time are cleaved off from fatty acid CoA, starting from the carboxyl end. The hydrogen produced from fatty acids are taken up the chain by hydrogen acceptors (e.g., FAD, NAD+) and produces a high yield of ATP. Acetyl CoA released can enter the tricarboxylic acid (TCA) cycle for ATP production, can form ketone bodies, or can be used for resynthesis of fatty acids.
For example, during the β-oxidation of palmitic acid (C16:0), through degradation of two carbon fragments, total ATP and net ATP generated is shown below.
a. Carbon-carbon cleavage
i. 7 cleavage (shown by red lines) = 5 ATP per each cleavage (1 FADH + 1 NADH)
b. Oxidation of acetyl CoA
i. 8 acetyl CoA units entering the TCA cycle = 12 ATP per acetyl CoA unit
Total ATP for 1 mole of palmitic acid (C16:0) = 35 ATP + 96 ATP = 131 ATP
= 7 cleavage points (7 × 5 = 35 ATP) + 8 acetyl CoA units (8 × 12 = 96 ATP)
Net ATP generated by 1 mole of palmitic acid (C16:0) = 35 + 96 − 2 = 129 ATP
(Two ATP used for initial activation and are then subtracted from 131 ATP)
Fatty acids of 16 C and 18 C are oxidized through the β-oxidation pathway. Other longer-chain fatty acids are oxidized in the peroxisomes. β-oxidation of unsaturated fatty acids occurs until the first double bond is reached. The double bonds are isomerized from cis to trans and then hydrolyzed by isomerase, ultimately yielding acetyl CoA.
Lipogenesis and Fatty Acid Synthesis
Lipogenesis is the process of synthesizing lipids as a means of storing chemical energy. Fat cells, or adipocytes, are dispersed throughout the body and are considered a long-term energy depot. Lipogenesis encompasses fatty acid synthesis (cytosol of hepatocytes and adipocytes), adipocyte uptake, and storage of lipids as the body’s “savings account.”
What Causes Fatty Acid Synthesis? Glucose is the key signal for fat storage (feasting). Excess energy (ATP) generated from glycolysis and the TCA cycle is taken up by the body to convert it to fat. As ATP levels increase beyond the cells’ requirements, the ATP begins to accumulate, which stimulates activity of the enzyme acetyl CoA carboxylase. Increased insulin concentrations are also required to stimulate acetyl CoA carboxylase activity.
Fatty acid biosynthesis begins with 2 C acetyl CoA (like β-oxidation). Acetyl CoA could come from fats, carbohydrates, or some amino acids. Fatty acid synthesis occurs in the cytosol (endoplasmic reticulum). The fatty acid chain is assembled in 2 C units (derived from acetyl CoA) by joining the carboxyl end of one fragment to the methyl tail of another, yielding palmitic acid (C16:0) as the end product.
Briefly, acetyl CoA carboxylase uses one ATP to combine one mole of acetyl CoA to form malonyl CoA. Fatty acid synthesis begins with the addition of carbon dioxide to 2 C acetyl CoA to form 3 C malonyl CoA. Acetyl CoA carboxylase is the enzyme needed for this step. Malonyl CoA reacts with acetyl CoA to produce a 5 C intermediate compound, which is decarboxylated to form a 4 C butyryl CoA. This in turn then combines with another malonyl CoA and is decarboxylated to form 6 C caproyl CoA . The step is continued by adding malonyl CoA and losing CO2 each time to produce 16 C palmitic acid as the eventual final product.
Most fatty acids are even numbered because each acetyl CoA, and subsequent malonyl CoA, is only two carbons in length. Each malonyl CoA adds two more carbons, and the resulting fatty acid is almost always evenly chained. Some very minor odd-numbered fatty acids (e.g., C17:0, C19:0) are present in animal tissues.
Fatty acid synthase is the enzyme involved in these steps. Palmitic acid (16 C) can be elongated to form 18 C stearic acid by elongases. Further formation of unsaturated fatty acid (oleic acid) C18:1 is by the introduction of double bonds by the desaturase enzyme. Mammals cannot insert double bonds beyond ∆-9 position. Therefore, linoleic (18:2 ∆-9,12) and linolenic (18:3 ∆-9,12,15) need to be provided in the diet and are called essential fatty acids. However, upon consumption, mammals can further desaturate and elongate the 18 C essential fatty acids to form longer chain 20 and 22 C fatty acids.
Storage and Mobilization of Lipids
Lipids are stored as TAG. The glycerol backbone of TAG is formed from dihydroxyacetone phosphate, produced from the preparatory phase of glycolysis (along with glyceraldehyde-3-phosphate). Glycerol is three carbons, and therefore, each carbon can bond together with a fatty acid. The bonding of the glycerol and fatty acids occurs mainly within the cytosol of hepatocytes or adipose tissue in ruminants (or the mammary gland in lactating animals). Once the TAGs have been formed, they are attached to lipoproteins and deposited in the blood for transport. The lipoprotein complexes are recognized by lipoprotein lipase, and the TAGs are removed and deposited as fat (e.g., fat pads in chickens, back fat in pigs).
Mobilization of lipids from storage sources for energy production is through the action of hormone-sensitive lipoprotein lipase, which releases free fatty acids and glycerol. Mobilization occurs during starvation (fasting), stress or increases in energy usage (e.g., disease). Hormones such as glucagon and epinephrine increase, and insulin is reduced under these conditions and stimulates the action of lipases (hormone sensitive).
In dairy cattle and in ewes during certain conditions such as negative energy balance, fat mobilization is at its peak and may exceed the rate at which acetyl CoA enters the TCA cycle. During such conditions, hepatic synthesis of ketone bodies (ketogenesis) occurs. The ketone bodies are acetoacetic acid, β-hydroxybutyric acid, and acetone. Ketosis is a metabolic disorder when excessive quantities of ketone bodies are produced and is also described in the chapter on bioenergetics (Chapter 17).
Ruminant Animals: Ruminant animals derive acetyl CoA directly from absorbed acetate (volatile fatty acid) rather than glucose. Citrate is permeable and is transported across the mitochondrial membrane and is converted to α-ketoglutarate with the production of nicotinamide adenine dinucleotide phosphate (NADPH). The α-ketoglutarate reenters mitochondria, while NADPH is used for fatty acid synthesis from acetate. It is a means for ruminants to conserve glucose and glucogenic precursors.
Key Points: Lipid Metabolism
Lipid Transport
- Chylomicrons enter the liver and are packaged into very-low-density lipoproteins (VLDL).
- VLDL delivers triacylglycerols (TAGs) from the liver to extrahepatic tissues. Once they unload the TAGs at the target tissues, their density increases and thus the LDL and HDL increase as well.
- LDL carries most of the cholesterol to tissues. Once they unload the cholesterol at the target tissues, their density increases, and thus HDL increases as well.
- HDL offloads all the remaining cholesterol and triglycerides to liver and is marked for excretion.
Lipid Storage
- For TAGs to enter the cells, lipoprotein lipase is required to hydrolyze them into fatty acids and glycerol again. The formation of TAGs requires a glycerol backbone, which can only come from glycolysis. Consequently, glucose is the key signal for fat storage.
Lipid Mobilization
- Mobilized lipids are used for energy production. Mobilization of fat out of adipose tissue requires another lipase. Hormone-sensitive lipase hydrolyzes TAGs and releases fatty acids and glycerol into blood circulation and provides other tissues with the substrate for energy.
β-Oxidation of Fatty Acids (Energy Production)
-
- Activation of fatty acid
- is done by acetyl CoA synthase to yield fatty acyl CoA
- is ATP dependent, needs two ATP
- Transportation into the mitochondria (carnitine needed)
- Degradation of two carbon fragments (β-oxidation) provides one NADH and one FADH per each acetyl CoA.
- Activation of fatty acid
Ketogenesis occurs under negative energy balance conditions in the liver.
- Excessive acetyl CoA accumulated from β-oxidation cannot be used in the TCA cycle; low incoming glucose due to starvation force the liver to make ketone bodies that can serve as an alternate energy source. However, continued synthesis leads to an accumulation of ketone bodies in the blood.
Fatty Acid Synthesis
- The location is cytosol.
- The starting material is acetyl CoA.
- The key enzymes are acetyl CoA carboxylase and fatty acid synthase complex.
- Starting with acetyl CoA (mainly from carbohydrates), two carbon units are added from the carboxyl to the methyl end.
- The cycle continues through the addition of malonyl CoA (building block for synthesis) and loss of CO2 up to 16 C.
- Further elongation and desaturation take place to form long-chain fatty acids. However, mammals cannot insert double bonds beyond ∆-9 position.
- Two fatty acids, linoleic and α-linolenic, are essential and should be included in the diet.
1. Why do most plant and animal fats have even-numbered fatty acids?
2. What are essential fatty acids and why are they essential?
3. What are the sites of fatty acid synthesis and oxidation in the cell?
4. What is the starting material for fat synthesis?
5. What are ketone bodies, and what is ketosis?
6. This lipoprotein is commonly referred to as “good” cholesterol.
7. This lipoprotein is commonly referred to as “bad” cholesterol.