XI. Proteins, Metabolism
All proteins in the body are in a state of constant flux, the size of the amino acid pool depends on a balance between synthesis and degradation. In this chapter, amino acid metabolism involving protein and nonessential amino acid synthesis and disposal of toxic ammonia is discussed.
New Terms
Citrulline
Deamination
Deoxyribonucleic acid (DNA)
messenger RNA (mRNA)
Ornithine
Protein turnover
Ribosomes
Transamination
transfer RNA (tRNA)
Urea
Uric acid
Urea cycle
Chapter Objectives
- To introduce the fate of absorbed proteins and synthesis of nonessential amino acids
- To discuss the process of detoxification of ammonia produced through nitrogen metabolism
The Fate of Absorbed Proteins
Absorbed proteins are used for anabolic purposes such as synthesis of nonessential amino acids, tissue protein synthesis, enzyme or hormone synthesis, deamination, or transamination.
Synthesis of Nonessential Amino Acids
The liver is the major site of amino acid metabolism. The liver has enzymes such as transaminases and is responsible for nonessential amino acid synthesis through a process called transamination. In this reaction, an amino group from one amino acid is transferred to an organic acid to form a new amino acid. Vitamin B6 (pyridoxine) is needed for transaminase activity.
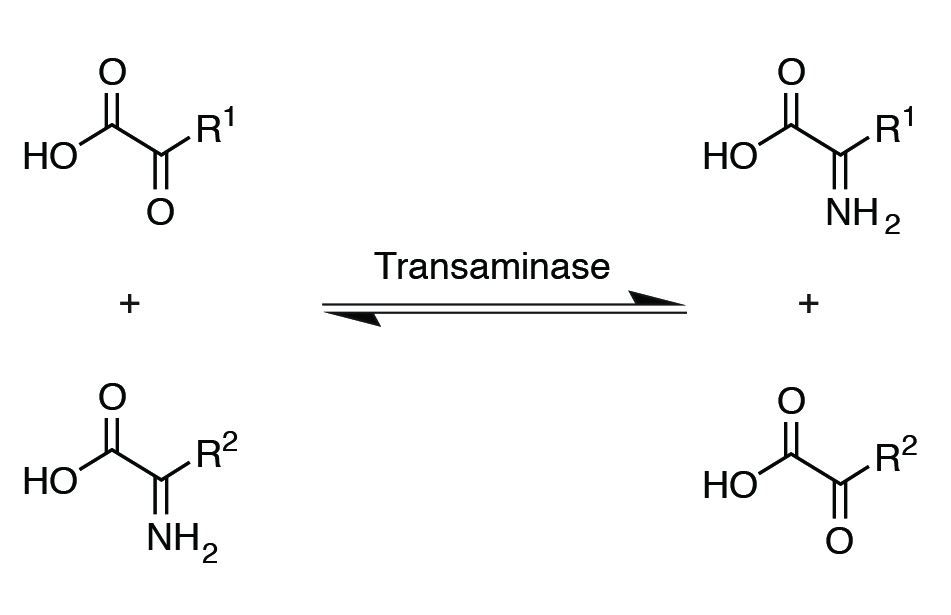
Transamination also provides a link between protein and carbohydrate metabolism, where certain amino acids can use their C skeleton for glucose synthesis. Deamination is the removal of amino groups from amino acids to form ammonia. This process is needed for getting rid of nitrogen from the animal’s body. After deamination or transamination, C skeletons are left and are used for making glucose, ketone bodies, or energy production.
The Fate of the Carbon Skeleton
- Oxidized for energy
- Used for glucose synthesis
- Used for ketone body formation
- Used for fat synthesis
All amino acids, except leucine and lysine, are glucogenic, meaning that they can use the C skeleton for glucose synthesis. Leucine and lysine are strictly ketogenic amino acids (forms ketone bodies) and can provide acetyl CoA as an energy source. Since both carbons of acetyl CoA are lost in the tricarboxylic acid (TCA) cycle, it cannot provide glucose. Some amino acids (isoleucine, phenylalanine, tyrosine, tryptophan) are both glucogenic and ketogenic.
All amino acids, except leucine and lysine, are glucogenic. Leucine and lysine are strictly ketogenic.
Some amino acids can be glucogenic or ketogenic.
Urea Cycle and Detoxification of Ammonia
The ammonia liberated from amino acid degradation is toxic to the central nervous and needs to be excreted or detoxified. Most mammals detoxify ammonia and excrete it as urea in the urine, while birds excrete it as uric acid (a white substance in the excreta). The detoxification of ammonia to form urea is brought about by the urea cycle through two tissues (liver and kidney; Figure 11.2).
Two nonprotein amino acids (amino acids not used for protein synthesis) involved in the urea cycle are ornithine and citrulline The first step in the urea cycle is the formation of carbamoyl phosphate through the condensation of ammonium ions with bicarbonate ions in the mitochondria of the liver.
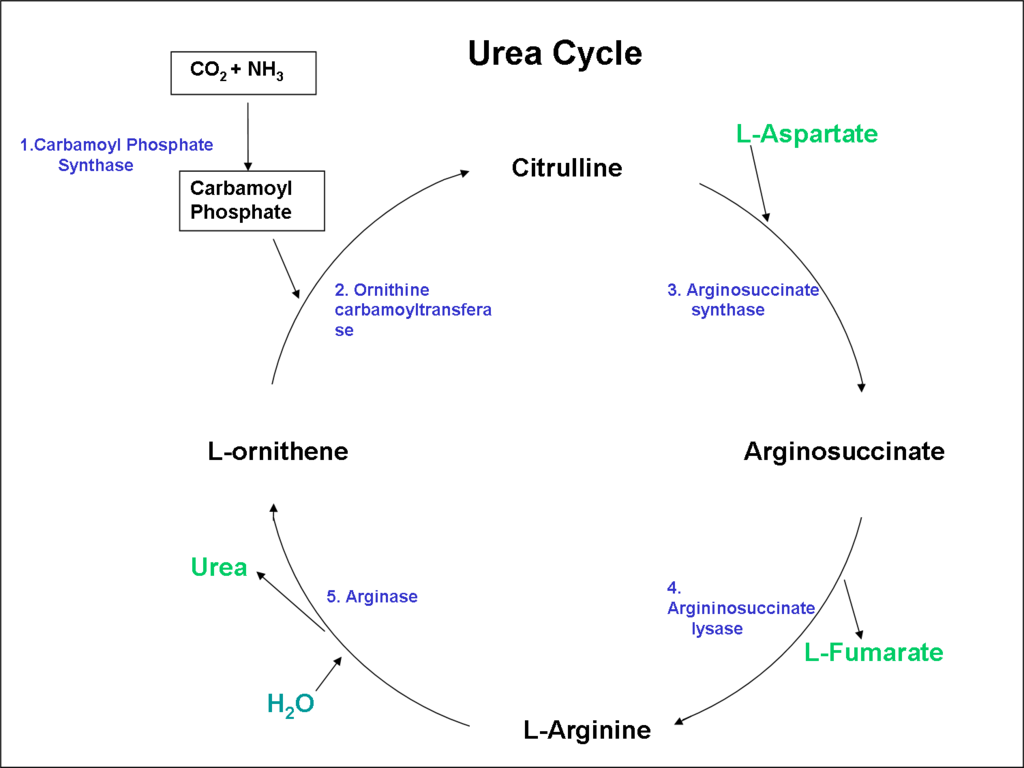
Ornithine reacts with a compound called carbamoyl phosphate and forms citrulline. Citrulline is easily permeable and gets into the cytosol and reacts with aspartate, forming argininosuccinate (2 ATP needed). The enzyme argininosuccinate lyase cleaves argininosuccinate into arginine and fumarate and fumarate enters the TCA cycle. Arginine is lysed into ornithine and splits urea off, producing ornithine, to start the cycle again. Hence arginine can be a nonessential amino acid but not available for protein synthesis.
The kidneys synthesize arginine from citrulline. The liver breaks down arginine into urea and ornithine.
Poultry cannot synthesize carbamoyl phosphate and hence they cannot make urea. Instead, glutamic acid, glycine, and methionine are used for uric acid synthesis. Hence poultry need high levels of methionine, arginine, and glycine in their diet for optimum production. Since the formation of urea through the urea cycle is ATP dependent, feeding animals poor quality or excess protein is energy demanding and can lead to environmental problems (e.g., air ammonia, groundwater pollution).
In the ruminant animals, rumen NH3s are sent to the liver via the portal vein and undergo the urea cycle back to urea and enter the blood, where they are eventually secreted in the urine or brought back into the digestive tract as an N source for the rumen microbes.
Types of RNA
- Messenger RNA
- Transfer RNA
- Ribosomal RNA
Protein synthesis occurs in every tissue of the body. Protein synthesis lies ultimately in the genetic code. The details of protein synthesis are beyond the scope of the current chapter and are explained in other biochemistry books.
Briefly, nucleic acids such as deoxyribonucleic acid (DNA; molecule that stores genetic information) and ribonucleic acid (RNA) consists of nucleotides. DNA contains the genetic code of the animal and is the blueprint of protein synthesis. DNA controls the formation of RNA (a template for transcription). There are three different types of RNA (ribosomal RNA, messenger RNA, and transfer RNA). All three are involved in protein synthesis. Ribosomal RNA is the part of the structure of ribosomes, which has three base codons that code for amino acids (site of protein formation). Transfer RNA picks up specific amino acids from cytoplasm and transports it to the ribosomes (workbench where proteins are made). Transfer RNA (tRNA) acts like an adapter during protein synthesis. Each tRNA carries a specific amino acid. Messenger RNA determines the sequence of amino acids (translation) in the protein formed. Both messenger RNA (mRNA) and tRNA are produced from the DNA template. The synthesis of each protein is controlled by a different mRNA. As the peptide chain is formed, an empty space cannot be formed, which limits the peptide chain formation and protein synthesis. All 20 amino acids are needed for protein synthesis. For example, lack of essential amino acid in the diet can stop peptide chain formation and protein synthesis and affect body weight gain and animal performance.
Protein turnover is a dynamic process involving continuous and simultaneous protein synthesis and protein degradation. The net rate of protein gain or loss is governed by the balance of synthesis and degenerative processes. Constant turnovers of proteins in the body and the loss of proteins, mainly in feces, are the basis for protein requirement. Even when an animal is not growing, it still has a protein requirement. The amount of protein needed in the diet depends on age, physiological (e.g., pregnancy, lactation) and pathological status, and quality of the protein supplied.
Key Points
- The liver is the major site of amino acid metabolism.
- Nonessential amino acids are synthesized through the process of transamination.
- Degradation of amino acid involves two processes (deamination and transamination).
- Deamination is the removal of amino groups from the C skeleton and the release of ammonia.
- Toxic ammonia is disposed through the urea cycle for detoxification of NH3 into urea (mammals) or uric acid (birds).
- Two important functions of the urea cycle are detoxification of ammonia (liver) and provisioning of arginine (kidney) to form urea and ornithine.
- Two nonprotein amino acids (ornithine and citrulline) are involved in the urea cycle.
- The C skeleton then can be used for energy, glucose, other amino acids, or ketone body synthesis.
- All amino acids except leucine and lysine are glucogenic.
- Four amino acids are both ketogenic and glucogenic (e.g., tyrosine, tryptophan, isoleucine, phenylalanine).
- Protein synthesis requires transcription and translation. The DNA template is transcribed into messenger RNA (mRNA), which in turn, serves as a template (translation) for protein synthesis.
- Transfer RNA (tRNA) carries the individual amino acid to the ribosomes and links together to form a polypeptide chain. Twenty individual amino acids are required at the same time to complete this task.
- There are constant turnovers of proteins in the body, and there is a loss of proteins in the body as well, mainly in feces.
- Protein need depends on age, physiological status, and quality of the protein supplied in the diet.
Review Questions
- What are the functions of ornithine and citrulline?
- In what form is nitrogen excreted in (a) swine and (b) chickens?
- Why do poultry have a greater need for arginine and methionine?
- In the urea cycle, carbamoyl phosphate condenses with ___.
- Citrulline and forms ornithine
- Ornithine and forms citrulline
- Arginine and forms citrulline
- Ornithine and forms arginine
- Proteins can be used for ___.
- Gluconeogenesis
- Ketogenesis
- Fat synthesis
- All of the above
- Two amino acids are strictly ketogenic. Which two?
- Which of the following molecules is responsible for carrying the specific amino acids to the ribosome for protein synthesis?
- tRNA
- mRNA
- DNA
- rRNA
- Differentiate between deamination and transamination.

