I. Introduction to Nutrition
New Terms
Acid Detergent Fiber
Ash
Crude Fiber
Crude Protein
Detergent Fiber System
Dry Matter
Ether Extract
Feed
Fundamental Nutrients
Neutral Detergent Fiber
Nitrogen Free Extract
Nutrient
Proximate Analysis
Chapter Objectives
- To introduce and discuss the basic concepts of nutrition and some basic nutritional terminology
- To introduce and discuss fundamental nutrients in animal diets
Concepts of Nutrition
Nutrition is a relatively new science. It is an applied science that encompasses the principles of other sciences, such as chemistry, biochemistry, and physiology.
Animal nutrition deals with the nutritional needs of food-producing, companion, or service animals. It is the science of preparation or formulation of feed for animals that produce food (e.g., meat, milk) or nonfood materials (e.g., wool). Animal nutrition also is an integrative science, as it deals with the different steps by which the animal assimilates feed, or food, and uses it for its growth, health, and performance (e.g., meat, milk, and egg production and service).
In addition to the health, welfare, or productivity of the animal, food animal nutrition is also very important due to economic (e.g., feed cost) and environmental aspects (manure and undigested, wasted nutrients, such as phosphorus and nitrogen, contaminating air, soil, and water), as well as nutritional quality (eggs, meat, milk).
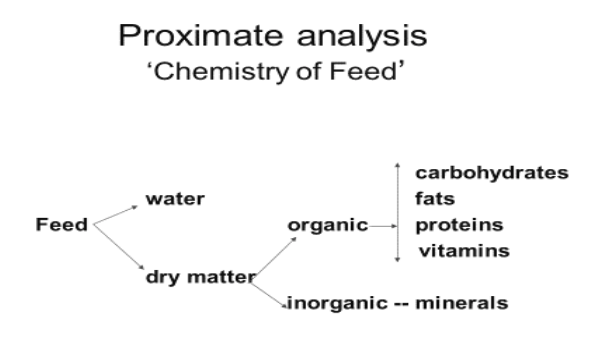
Nutrients are chemical elements or compounds present in feed that support health, basic body maintenance, or productivity. Fundamental nutrients include water, carbohydrates, protein, fat, vitamins, and minerals.
Why Is Nutrition Important in Livestock?
Nutrition is important for all organisms. However, in food-producing animals, it is especially important due to the nature of the production systems (e.g., confinement), the economics of production, or the products (e.g., meat, eggs, milk) generated.
Feed nutrients, such as nitrogen and phosphorus, are lost into the environment through manure, which if not managed properly, can lead to environmental pollution. The emission of methane and nitrous oxide from manure is also to some extent dependent on the nature of feed being fed to livestock. Use of good-quality feeds with high digestibility will minimize or reduce environmental pollution.
Feed represents the major expense for raising food animals. For example, feed amounts to more than 65% of the expense in swine or poultry production systems. As world population increases, there is an additional demand for food, land, and energy. As a result, feed production with limited resources will be a challenge in the context of sustainability.
Consumers’ perception of the effect of diet on health has increased markedly over the past two decades. This perception has an impact on consumer food choices, especially with regards to certain nutrients in animal products (e.g., saturated fats, cholesterol). Therefore, nutrition is important for producing health-promoting foods for human consumption.
Improper nutrition (under- or overfeeding) can affect animal health. Balanced nutrition can enhance immune health, welfare, productivity, and longevity. Overall, the nutrition of livestock is very important due to their dependence on humans, especially when food animals are raised in confinement. It is also important for economic reasons, to produce human food with limited resources, and to enhance animal productivity, health, and welfare.
Why Nutrition is Important
- Dependence on humans (e.g., confinement)
- Economics
- Environmental protection
- Enhancement of food production with limited resources
- Human health-promotion and food quality enhancements
- Animal health and welfare
Nutrient Analysis of Feedstuffs
The 19th century had a significant impact on modern animal nutrition. Developments during this period include the introduction of fundamental nutrients and the separation of feed into protein, fat, and carbohydrate components. In this respect, proximate analysis, a combination of analytical procedures devised more than 100 years ago by German scientists at the Weende Experiment Station (also known as Weende analysis), paved the way for estimating the nutrient content of feed samples. Although detailed knowledge of different analytical procedures is not required, familiarity with different basic feed analyses will enhance learning and understanding of animal nutrition.
Why Perform Nutrient Analysis of Feedstuffs?
Animal nutrition is the science of feed preparation (formulation) and feeding to meet the needs of animals at different phases of growth, or life stages. Therefore, nutritionists need to know the nutrient components of the feed or the raw materials used in ration formulation. Nutrient analysis serves as a system to analyze the feed and the needs of the animal, enabling producers to optimize nutrient utilization in feed and helping researchers relate to animal performance, tackle issues of underperformance, and reduce food production costs.
Reasons for Nutrient Analyses in Feed
- Ration formulation and feeding
- Trouble shooting
- Economics
Sampling Feed for Analyses
Modern chemical methods and equipment need only a small amount of the feed (2 to 10 g) for analyses. Therefore, sample materials collected and prepared for analyses should represent the best reasonable estimate of the total feed fed to animals. Sample integrity during preparation (e.g., grinding, drying), storage (e.g., temperature), and transportation should be considered. The frequency of feed analysis depends on batches of feed made, variability of feed sources (e.g., cultivar, location of growth), and cost of analyses. Several core samples should be taken, combined, ground, and subsampled. Avoid taking a sample directly from outside of a bale (use common sense)! Weather patterns should also be considered, as they can affect the moisture content of the sample.
Analytical Methods
Traditionally, feedstuffs are subjected to different protocols of laboratory analyses (wet chemistry) for nutrient profiling. These analytical procedures are specific for a given element (e.g., N), compound, or group of compounds. Chemical methods often employ drastic degradation of the sample with different acids or other solvents and may not be true estimates of an animal’s ability to utilize them efficiently. However, considering the time and cost of other methods using live animals (e.g., explained in chapter 20) that provide more accurate estimates, laboratory analyses are used widely to get a head start.
Proximate Analysis
Proximate analyses are a combination of analytical procedures developed in 1865 by Wilhelm Henneberg and Friedrich Stohmann at the Weende Experiment Station in Germany. They are based on the elimination of water from the feed (as shown later) and then the determination of five proximate principles in the remaining dry matter (DM). They are as follows, and their names refer to specific proximate principles:
Dry Matter
The determination of dry matter (DM) is the most common procedure carried out in nutrition laboratories because plant feedstuffs may vary in water content. The amount of water content must be known to permit comparisons of different feeds.
DM is determined by drying the test material at 105° C overnight in an oven. DM is then determined by the following calculation:
dry weight / fresh weight (also called as-fed weight) * 100 = % DM.
Most feeds are around 90% DM, and silages are about 30% to 35% DM. Possible errors in DM analyses include loss of volatile fatty acids (VFAs), essential oils, lactic acid in silage, or any other fermented products. Moisture can also be determined by moisture meters, but results are not as precise as those obtained by drying testing materials in the oven. Freeze-drying or drying at lower temperatures can minimize errors.
The following is an example of DM calculation on a batch of corn silage samples:
Fresh (as-fed) weight = 2 kg
Dry weight = 0.7 kg
DM % = (0.7 kg/2.0 kg)*100 = 35%
A dry matter (DM) test estimates moisture.
The higher the DM, the lower the moisture.
Crude Protein (CP)
The procedure to estimate crude protein was developed by a Danish chemist, Johan Kjeldahl and is commonly known as “Kjeldahl” procedure. The Kjeldahl analysis depend on the measurement of nitrogen (N) in the test material. To convert the measured N content of the test material to crude protein, a calculation factor of 6.25 (N x 6.25) is applied. This is based on the fact that all proteins contain about 16% N(100/16 = 6.25) or 16 g of N comes from 100 g protein, or 1 g of N is associated with 100/16 = 6.25 g of protein.
NITROGEN (N) * 6.25 = CRUDE PROTEIN (CP)
The following is an example of crude protein calculation on a batch of soybean meal samples:
Nitrogen content = 7.35 g
Crude protein = 7.35 × 6.25 = 45.9 g
The Kjeldahl procedure measures nitrogen, not protein.
This process of nitrogen determination involves boiling the dried samples in 36 N sulfuric acid (H2SO4). This will convert nitrogen to ammonium sulfate ([NH4]2SO4). The mixture is then cooled and neutralized with 12 N sodium hydroxide (NaOH). This will release ionized ammonium. The sample is then distilled, and the distillate containing the ammonium is titrated with 0.02 N sulfuric acid. This analysis is accurate and repeatable but time consuming and involves the use of hazardous chemicals. The information obtained on N content and hence CP content is of limited use to nonruminants, such as pigs and poultry, as it does not indicate the quality of the protein, but it is applicable to ruminant animals that can efficiently utilize all forms of N.
A possible error in the Kjeldahl method is assuming all nitrogen presented in the sample is in protein form. This assumption is not necessarily true because nitrogen could be in nucleic acids (ribonucleic acid (RNA) or deoxyribonucleic acid (DNA)) or can exist as nonprotein nitrogen, such as urea.
Ether Extract
Ether-soluble materials in feed include different organic compounds that are soluble in organic solvents. In animal feeds, ether extract may include fats, fatty acid esters, and fat-soluble vitamins and hence are often referred to as crude fat. The primary goal of ether extracts is to isolate the fraction of the feedstuff that has a high caloric value. A portion of the dried feed sample is boiled in ether (organic solvent) for four hours. Since fats are soluble in ether, ether extract is equivalent to fat. Provided the ether extract contains fats and fatty acid esters, this approach is valid. However, in samples that contain high levels of other compounds soluble in organic solvents, such as plant waxes or resins, it may not give a true estimate of feed caloric value. However, this error is generally small in typical animal feedstuffs. Overall, this test does not indicate anything about the quality of the fat in the feed.
The Ether Extract procedure assumes substances soluble in ether are fats.
Ash
Ash is the residue remaining after all the organic nutrients have been burned off or oxidized completely in an oven at 500° to 600° C for two to four hours. Nutritionally, ash values have little importance, although high values may indicate contamination (e.g., soil) or dilution of the feed sample with limestone or salt. Ash values obtained are cumulative of all the mineral elements combined together. High temperatures used for burning may cause loss of some volatile elements such as chloride, zinc, selenium, iodine, and so on. Consequently, ash values can underestimate mineral contents. However, this error is small. Identifying individual minerals may be more meaningful and useful. If ash values are not very useful, why obtain them? They allow for calculations of nitrogen-free extract compared to DM (see later).
An ash test measures inorganic compounds in feed.
High ash values indicate feed contamination.
Crude Fiber
Crude fiber estimates the indigestible fraction of feed or those fractions of the feed that are fermented in the hindgut by microbes. Crude fiber includes different insoluble carbohydrates that are associated with the cell wall of plants and are resistant to the action of digestive enzymes. Crude fiber is made up of plant cell structural components, including cellulose, hemicellulose, lignin, and pectin. For nonruminant animals, crude fiber is of little value energy-wise. However, it is important for maintaining hindgut health and microbial population. Crude fiber is important in the diets of ruminant animals, which can ferment a large portion of it. Crude fiber is described in detail below.
Crude fiber measures fermentable components of the feed.
Crude fiber has little energy value but is important for gut health in pigs and poultry.
Ruminant animals can ferment a large portion of crude fiber.
To determine crude fiber in feed, a sample is dried, boiled in weak sulfuric acid (1.25% H2SO4), and filtered. The residue is boiled in a weak alkali (1.25% NaOH) and filtered, and the remaining residue is dried and ashed. The difference between the filtered dried sample and ash is crude fiber. The two boiling processes simulate the pH conditions of the digestive tract, acidic in the stomach and alkaline in the small intestine. However, the enzymatic digestion in the digestive tract is not simulated in the procedure.
Crude fiber tests underestimate true fiber in feed.
A major problem with this procedure is that the acid and base solubilize some of the true fiber (particularly hemicellulose, pectin, and lignin), and some cellulose is partially lost too. Hence crude fiber underestimates true fiber in the test material. The number, or value, obtained in this procedure, therefore, is practically meaningless. Most laboratories have phased out the crude fiber term and replaced it with the detergent fiber system (discussed in detail later)
Nitrogen-Free Extract
The term nitrogen-free extract (NFE) is a misnomer, as there is no nitrogen or extraction process in this procedure. Nitrogen-free extract is not determined analytically in the laboratory, as shown below. NFE supposedly represents the soluble carbohydrates of the feed, such as starch and sugar, and is the difference between the original sample weight and the sum of the weights of moisture (water), ether extract, crude protein, crude fiber, and ash. Therefore, it accumulates the errors of the other analytical systems. It is an overestimate of true NFE.
% NFE = (% DM − (% ether extract + % crude protein + % ash + % crude fiber)
Nitrogen-free extract is a calculated value and not an analyzed value.
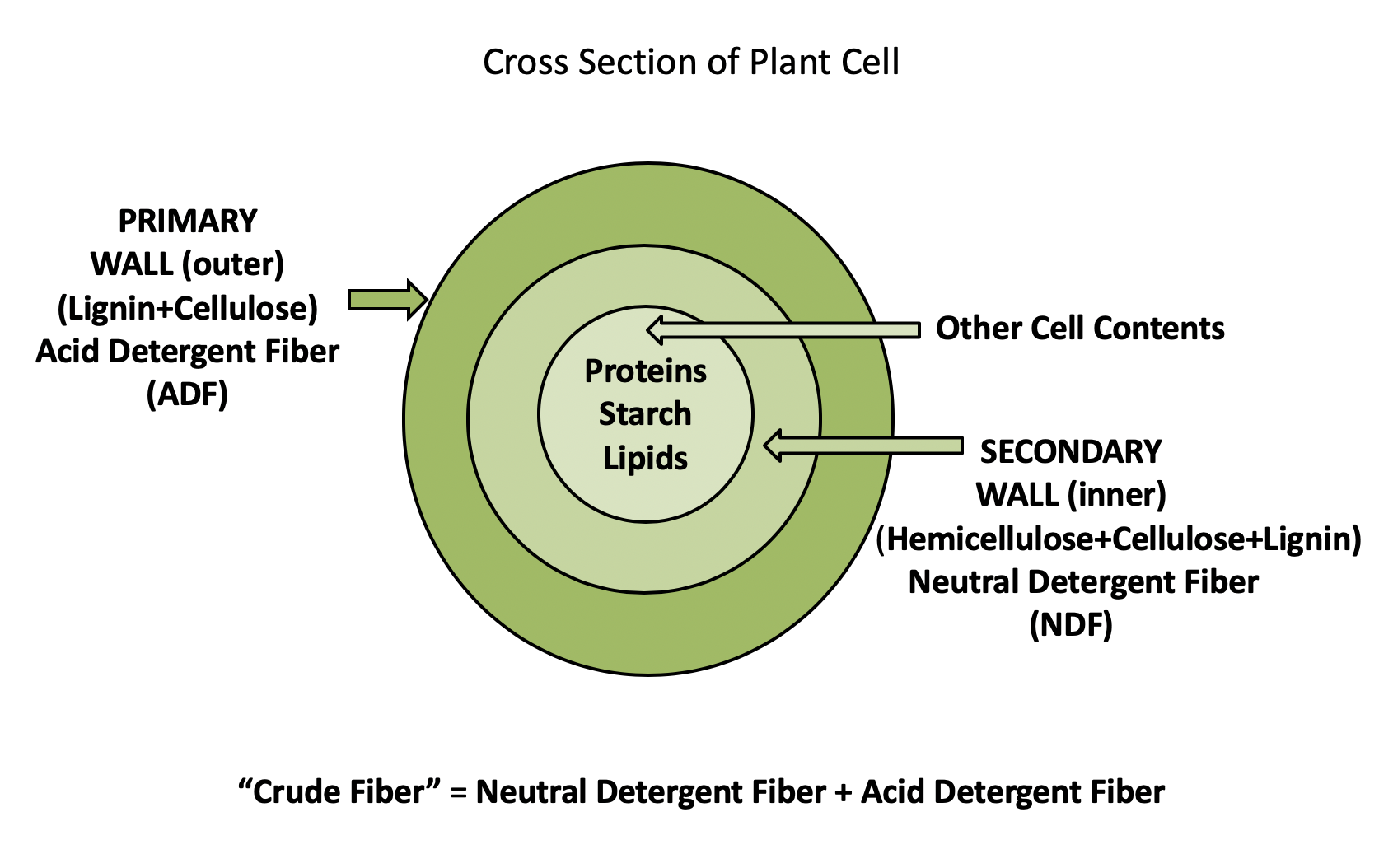
“All Fibers Are Not Created Equal”
Peter J. van Soest (1982) improved methods of crude fiber analyses into the detergent fiber system. The concept behind the detergent fiber system is based on the fermentability or digestibility of fiber. Accordingly, plant cells can be divided into cell walls (which contain hemicellulose, cellulose, and lignin and are less digestible) and cell contents (which are mostly digestible, such as starch and sugars) (shown below in detail).
Use of these methods allows plant components to be divided into neutral detergent fiber (NDF) and acid detergent fiber (ADF).
The detergent fiber system includes neutral detergent fiber and acid detergent fiber.
NDF contains the major cell wall components, such as cellulose, hemicellulose, and lignin. It may also contain other very important components, such as cutin, and some proteins too. Hemicellulose, cellulose, and lignin are indigestible in nonruminants, while hemicellulose and cellulose are partially digestible (fermentable) in ruminants.
NDF fractionation is determined by boiling feed samples for one hour in a solution containing sodium lauryl sulfate and ethylene diamine tetra acetic acid (EDTA) at pH 7.0. This detergent extracts soluble components of the feed (protein, sugars, lipids, and organic acids), and the nonsoluble material is called NDF.
NDF = Hemicellulose + Cellulose + Lignin
Acid detergent fiber is an estimate of cellulose + lignin in the feed sample. Hemicellulose, therefore, is estimated as NDF − ADF. This is not a perfect system, as there are contaminants in both ADF and NDF terms. ADF does the best job of describing the portion of feed it is designed to estimate (i.e., cellulose + lignin). The ADF and NDF terms have now largely replaced the crude fiber term. By using this method, we can better predict the digestibility of forages for animals. Nowadays, most laboratories use NDF and ADF analysis instead of crude fiber.
Key Points
- The broad classifications of nutrients are water, protein, fat, carbohydrate, minerals, and vitamins. These classifications are so broad that analysis of these has limited value.
- For analysis, feed should be sampled as many times as possible and then dried, ground, and mixed for subsampling.
- Drying is used in the first step of proximate analysis to determine the water content of a feedstuff. Some components of a feed may be lost through volatilization at this time. Usually, this is a small error.
- To reduce damage to feed, alternatives to drying include freeze-drying or drying at lower temperatures (i.e., 55° C)
- Ether extract (EE) is determined by extracting the dried sample in organic solvent (ether). It represents the fat content in the sample. It assumes all the substances soluble in ether are fat, which is not true.
- Crude protein (CP) is determined by the Kjeldahl method. It analyzes the N content of a diet and calculates protein using the assumption that all protein is 16% N. The problems with this are that some proteins are not 16% N and some feed constituents that contain N (i.e., urea, DNA, RNA) are not proteins.
- Ash is used to determine mineral content. It provides no information on actual amounts of individual minerals. It provides an estimate of the total inorganic component of the diet, which is often interpreted as contamination. Many feed tags indicate a maximum limit for ash as an index of quality.
- Crude fiber (CF) is an estimate of the cell wall constituent of a feed. Ideally, it should represent cellulose, hemicellulose, and lignin; however, the process of digesting feed with weak acid then weak base solubilizes some of these components (especially lignin and hemicellulose), and as a result, CF underestimates true fiber. It is the major limitation of the proximate analysis system.
- Nitrogen-free extract (NFE) is designed to provide an estimate of water-soluble polysaccharides (sugars, starch) and is calculated by the difference between the original sample weight and the sum of weights of moisture (water), ether extract, crude protein, crude fiber, and ash. Therefore, it accumulates the errors of the other analytical systems. It is an overestimate of true NFE.
- Van Soest developed improved methods of fiber analyses (the detergent fiber system). Acid-detergent fiber (ADF) is an estimate of cellulose + lignin, whereas neutral detergent fiber (NDF) is an estimate of cellulose + hemicellulose + lignin. Hemicellulose therefore is estimated as NDF − ADF. This is not a perfect system, as there are contaminants in both ADF and NDF terms. ADF does the best job of describing the portion of feed it is designed to estimate (i.e., cellulose + lignin). The ADF and NDF terms have now largely replaced the crude fiber term.
Review Questions
- How would you define “nutrition”?
- Why is nutrition important in today’s livestock production?
- What are the six major classes of nutrients?
- Proximate analysis of a feed includes the following tests:
- Crude protein (CP) is determined by the Kjeldahl method. It analyzes the content of _____ in the diet.
- Nitrogen
- Minerals
- Protein
- Water
- Ether extract is determined by extracting the dried sample in organic solvent (ether). It represents which component of the feed sample?
- As the dry matter content of a feed increases, the moisture content
- Increases
- Decreases
- Remains the same
- Among the different proximate analyses, this is a calculated value
- Dry matter
- Crude protein
- Crude fiber
- Nitrogen free extract
- This test measures the inorganic component of feed in proximate analysis
- Ether extract
- Moisture
- Crude fiber
- Ash
- A researcher conducted nitrogen (N) analysis on an unknown feed sample and was
found to be 7.0 g. The crude protein (g) content of the feed sample is calculated as follows:- 7.0 + 6.25
- 7.0 – 6.25
- 7.0/6.25
- 7.0 x 6.25
- Select the component of forage that is NOT a part of neutral detergent fiber (NDF)
- Starch
- Hemicellulose
- Lignin
- Cellulose
- Differentiate between neutral detergent fiber (NDF) and acid detergent fiber (ADF).