VI. Lipids, Structure
This chapter provides an introduction and discussion of lipids (fats) that are important in the nutrition of food-producing animals. After carbohydrates, lipids serve as a major source of energy in animal diets.
Cholesterol
Conjugated linoleic acid
Essential fatty acid
Fatty acid
Glycerol
Lipid
Monounsaturated fatty acid
Omega-3 fatty acid
Omega-6 fatty acid
Polyunsaturated fatty acid
Saturated fatty acid
Triglyceride
Chapter Objectives
- To present the chemical structure of lipids and fatty acids of importance in animal nutrition
Lipid Structure
What Are Lipids?
Lipids (also known as fats) are components of plant (e.g., vegetable oils) and animal tissues (e.g., meat, eggs, milk). On a physical nature, lipids are relatively insoluble in water and are soluble in organic solvents, such as hexane, ether, and chloroform.
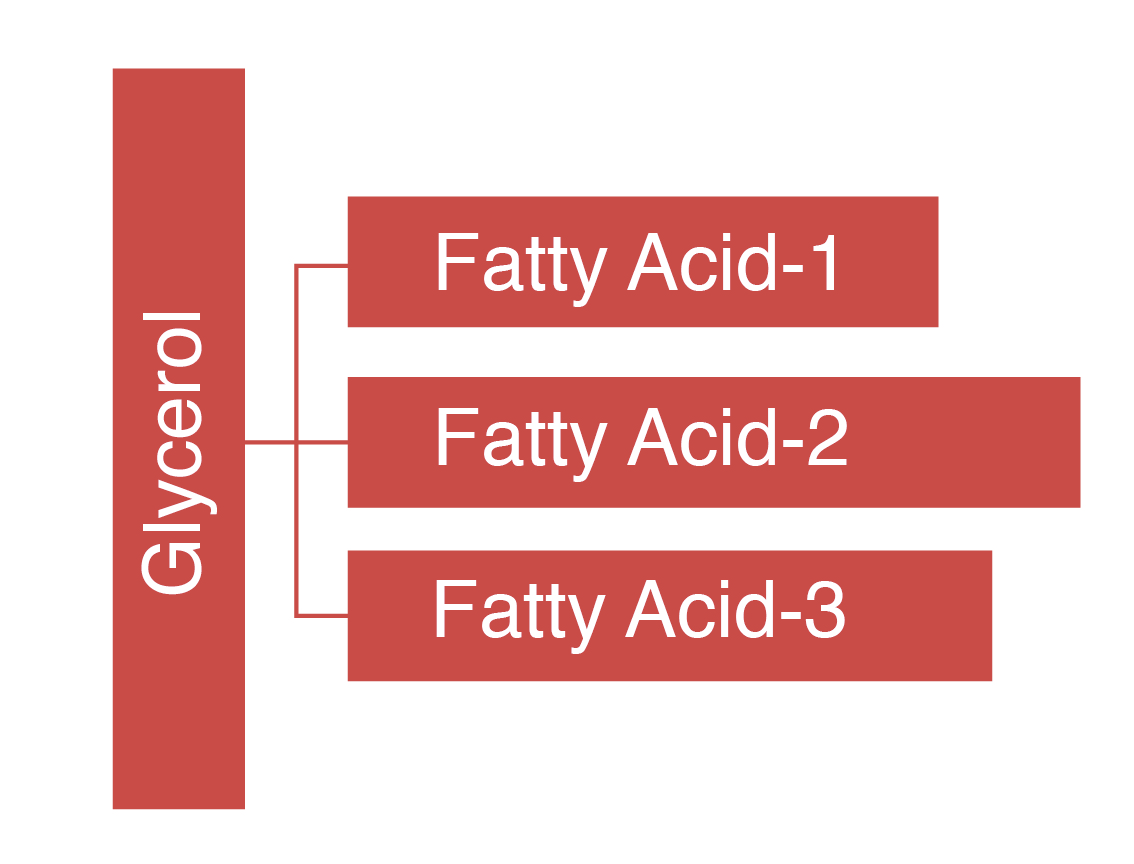
Chemically, lipids are organic compounds and esters of fatty acids and glycerol (a 3 C compound) or some other alcohol.
Lipid Classifications
-
- Simple lipid = Esters of fatty acid with alcohol, e.g. 1 glycerol + 3 fatty acids (commonly called triglyceride or triacylglycerol)
- Compound
- Glycolipid
- Lipoproteins
- Phospholipids
- Derived Lipids
Simple lipids like triglycerides are more common and are an important component in animal rations (e.g., vegetable oil and animal fats such as tallow or lard).
Compound lipids are composed of a lipid plus a nonlipid molecule (e.g., protein). Lipoprotein (lipid + protein) are examples of compound lipids and are used for lipid transport (like a courier). Within the animal body, compound lipids are more important in physiology and metabolism (e.g., lipid transport, phospholipids as part of cell membranes).
As implied by their name, derived lipids originate from simple or compound lipids through hydrolytic processes. Examples of derived lipids include sterols, fatty acids, and fat-soluble vitamins.
Why Add Fats to Animal Diets?
Nutritionally, fats are excellent sources of energy and are essential to the survival of animals. Fats are the sole source of essential fatty acids (those that cannot be made by the body) for animals. Fats can also provide fat-soluble vitamins. However, this role is very minimal in livestock as feeds are supplemented with vitamins.
As the fat content of the diet increases, the energy density of the diet goes up.
Physically, the addition of fats is associated with the improvement of feed quality, the reduction of dust in feed, the reduction of feed particle separation during processing, an increase in palatability, an increase in digestive lubrication (i.e., emulsification and rate of passage), and an increase in feed digestibility.
Fatty Acids: What Are They?
Fatty acids are the main players in lipid nutrition. This is due to their diversity in structure, composition, and metabolizability. The molecular composition of a fatty acid includes a hydrophilic carboxyl group (−COOH) and a hydrophobic methyl group (−CH3) at opposite terminals of a hydrocarbon backbone (see Figure 6.1).
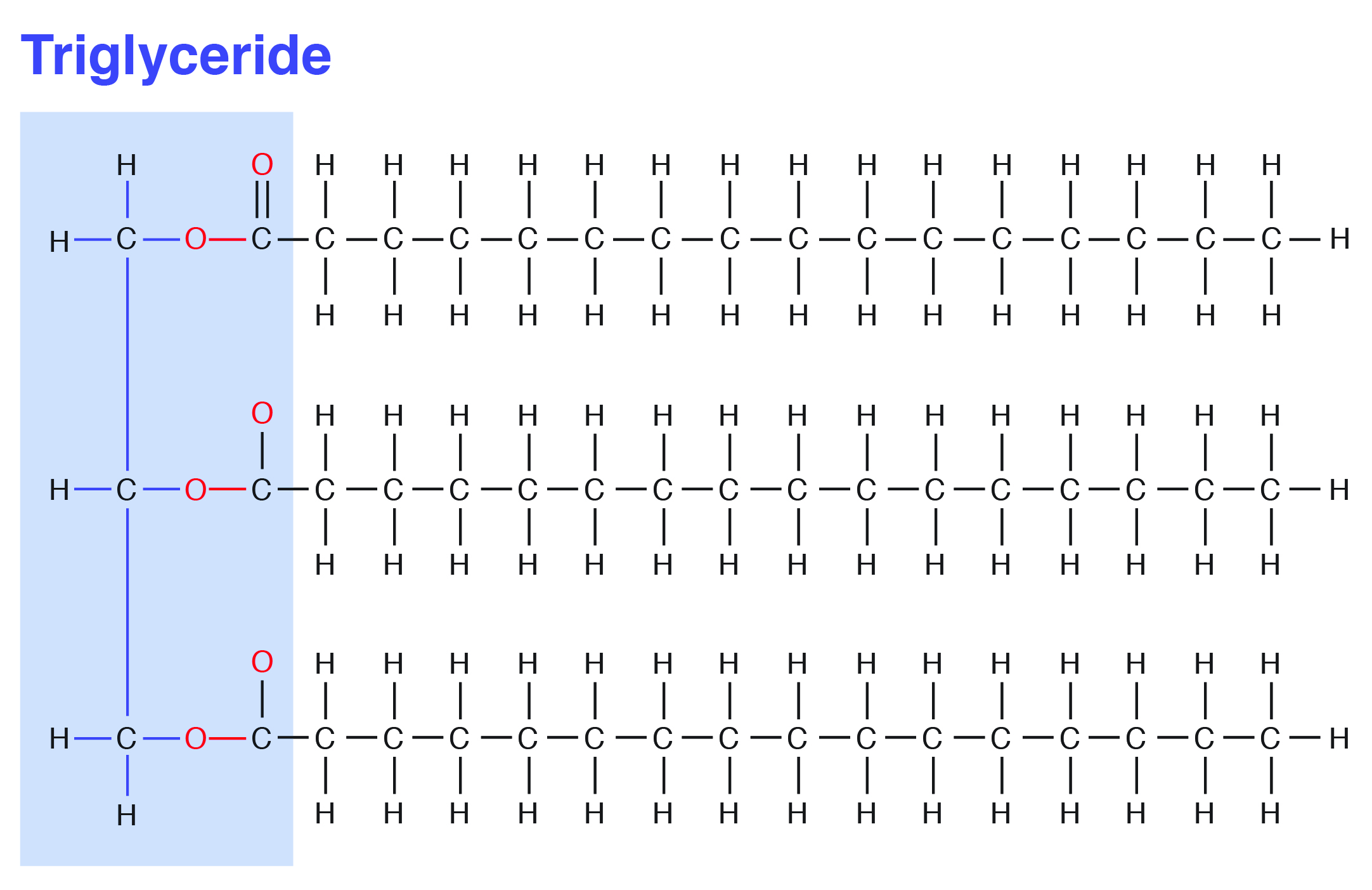
In most cases, three fatty acids are attached to the glycerol molecule and are called triacylglycerol. The three fatty acids in triacylglycerol can differ in chain length (i.e., total carbons in the fatty acid molecule) as well as in the number of double bonds.
A schematic representation of a triacylglycerol structure with three fatty acids on a glycerol backbone is shown below.
Fatty acid composition and structure determine the physical property and nutritional quality of fats. For example, when there is a predominance of saturated fats in the triacylglycerol, fat tends to solidify (e.g., fat around a piece of meat), and when there is a predominance of unsaturated fats, fat tends to liquefy (e.g., salad oil).
Physical Properties: Fatty Acids
- An increase in saturation makes fats more solid.
- An increase in unsaturation makes fats more liquid or decreases their melting point.
Fatty acids are classified into three families based on the presence (or absence) of double bonds in the hydrocarbon chain. These include saturated fatty acids, monounsaturated fatty acids, and polyunsaturated fatty acids (PUFAs).
Acids
- Saturated = no double bonds
- Unsaturated = presence of double bonds (could be one or two)
- Polyunsaturated = more than two double bonds
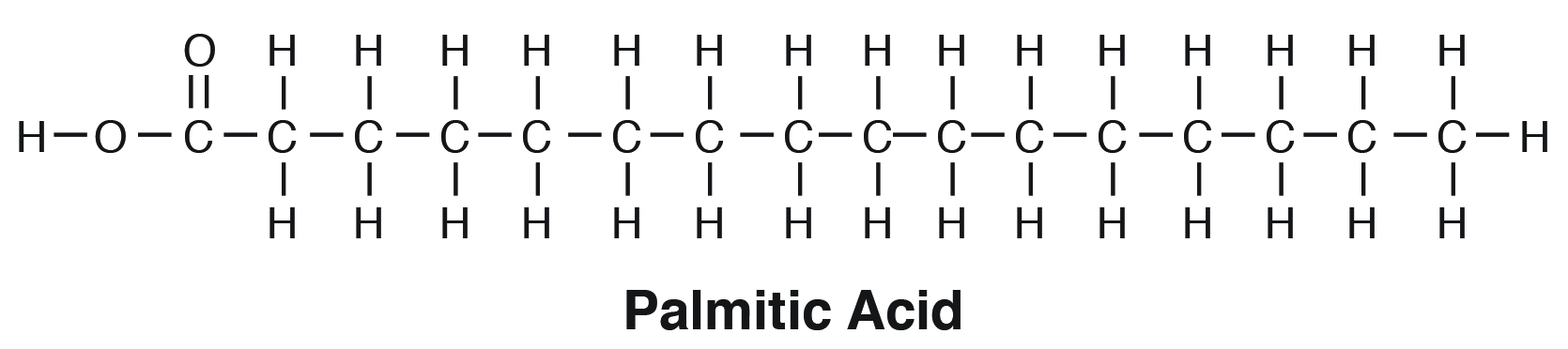
Saturated fatty acids are “saturated” with hydrogen or straight chains with no double bonds (e.g., palmitic acid, C16:0). When there is a predominance of saturated fats in the glycerol moiety, the triacylglycerol tends to be solid. This is because due to their straight chain nature, they tend to “pack” very tightly in the membrane (e.g., tallow or beef fat; Figure 6.2).
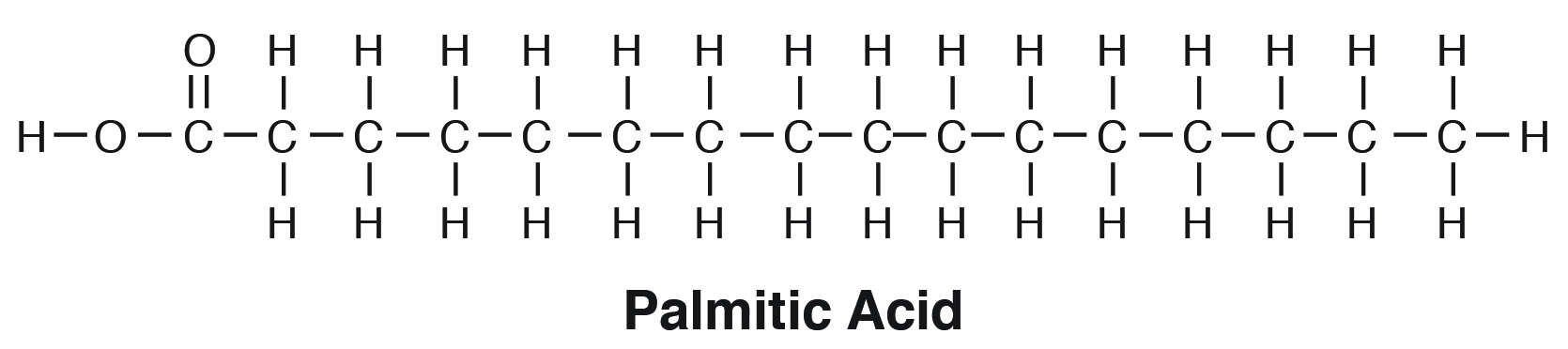
Unsaturated fatty acids contain one or more double bonds between adjacent carbon atoms in the hydrocarbon chain.
Unsaturated fatty acids may be either mono (one double bond) or polyunsaturated (more than two double bonds). When there is a predominance of unsaturated fats, the triglyceride tends to be liquid because unsaturation gives a “bend” in their structure and they cannot pack as tightly as saturated fats (e.g., vegetable oil).
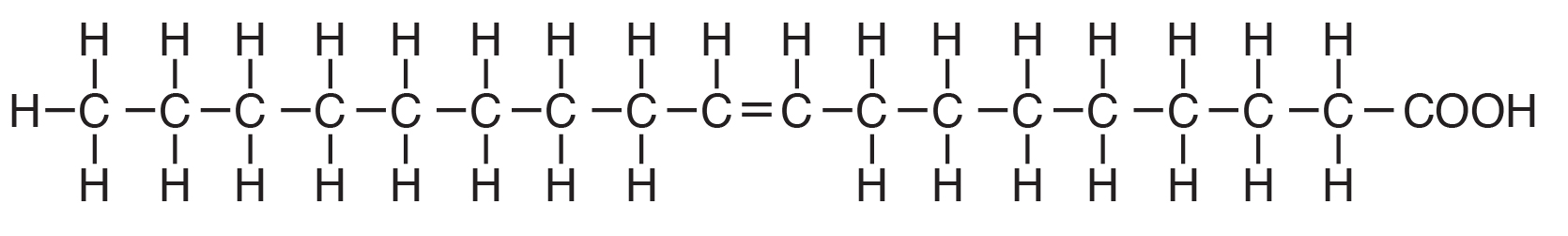
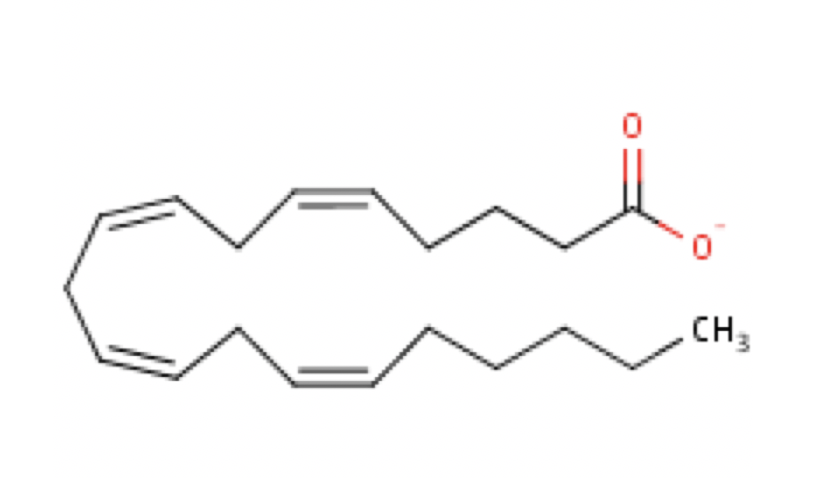
Polyunsaturated fatty acids are commonly called “PUFA” and contain two or more double bonds. Due to these extra double bonds, PUFAs tend to be more “round” when compared to the straight chain structure of a saturated fat (e.g., Figure 6.2 vs. Figure 6.4). These double bonds also change the physical nature of the fat, making it more liquid than straight chain saturated fat. In addition to the number of double bonds, the position of double bonds in the carbon-carbon chain is also important in nutrition and in the metabolism of lipids; this is explained below.
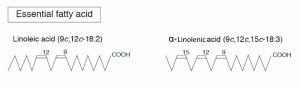
Nutritionists designate the term omega (ω) or “n” to denote the position of double bonds in the carbon chain in a PUFA. The omega carbon is the first carbon with a double bond counting from the methyl end (CH3) of the carbon chain. The two PUFA classifications are omega-6 (also called n-6, or ω-6) or omega-3 (n-3, or ω-3). For instance, omega-3 fatty acid will have the first double bond at the third carbon when counted from the methyl (CH3) end (Figure 6.5a) and omega-6 fatty acids will have the first double bond at the sixth carbon when counted from the methyl (CH3) end (figure 6.5b). The locations of the double bonds are also indicated by the Greek letter Δ, “delta,” in some chemistry or biochemistry textbooks. The delta term denotes the position of double bonds from the carboxyl end. However, the term omega, or “n,” is the one that is commonly used by nutritionists.
Two Types of PUFA
- Omega-6 (n-6, or ω-6) fatty acid
- Omega-3 (n-3, or ω-3) fatty acid
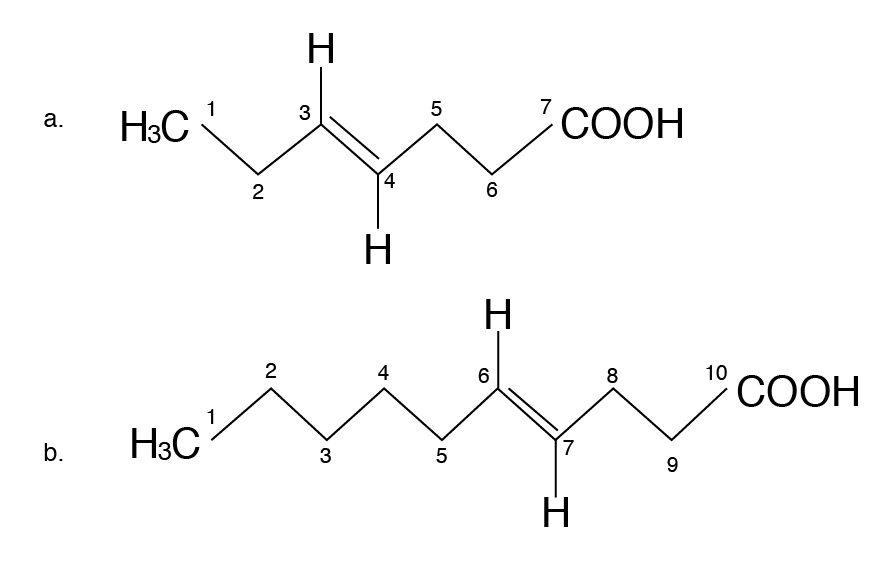
Essential Fatty Acids
In nonruminant, or monogastric, animals such as pigs, two fatty acids (α-linolenic acid, C18:3 n-3) and linoleic acid (C18:2 n-6) have to be supplied in the diet and are called essential fatty acids. This essentiality is due to the inability to insert double bonds at the third and sixth carbon from CH3 end in n-3 and n-6 locations. In addition to these two essential fatty acids, carnivores such as cats need arachidonic acid (C20:4 n-6) in their diets.
In nutrition, the term “essential” means animals cannot synthesize it to meet their requirements. Essential fatty acids include the following:
- Linoleic acid (C18:2 n-6)
- Linolenic acid (C18:3 n-3)
- Arachidonic acid (C20:4 n-6; in true carnivores, e.g., cats)
Fatty Acid Nomenclature
Fatty acids are commonly expressed by their trivial names (e.g., linoleic acid) or their associated shorthand notations (C18:2 n-6). The shorthand nomenclature of a fatty acid includes the number of carbon atoms and double bonds. For instance, in linolenic acid, C18:2 n-6 stands for 18 carbon atoms and two double bonds, of which the first double bond is at the sixth carbon atom from the methyl carbon. Some of the common fatty acids in animal foods, such as chicken or pork, and their trivial names and shorthand notation are shown in Table 6.1.
Cis and Trans Fatty Acids
Unsaturated fatty acids can form geometric isomers, with either cis or trans, depending on the stereo-conformation of groups around a double bond. Most natural fatty acids of animal and plant origin are of the cis type, whereas those of bacterial origin contain both cis and trans types.
| Palmitic Acid | C16:0 |
| Palmitoleic | C16:1 |
| Stearic acid | C18:0 |
| Oleic acid | C18:1 |
| Linoleic acid | C18:2 n-6 |
| Linolenic acid | C18:3 n-3 |
| Arachidonic acid | C20:4 n-6 |
| Docosahexaenoic acid | C22:6 n-3 |
For example, conjugated linoleic acid (CLA) is a trans fatty acid present in cow’s milk or other ruminant food like beef and is produced by rumen microbes during the biohydrogenation process. In CLA, the two double bonds lack a methylene group separating them, have a conjugated arrangement, and are called natural trans fats. Trans fats such as CLA have received considerable attention due to their several health-promoting (e.g., anticancer, immune health enhancing, lean body mass enhancing) effects. There are other trans fats that are produced during the hydrogenation process (the addition of hydrogen) when liquid vegetable oil is made into solid fats such as margarine. These are synthetic trans fats and have different health effects when compared with “natural” trans fats such as CLA.
∆-9 (counted from carboxyl end of the hydro carbon chain is shown as the position of the first double bond in linoleic (18:2 ∆-9,12) and linolenic (18:3 ∆-9,12,15) acid.
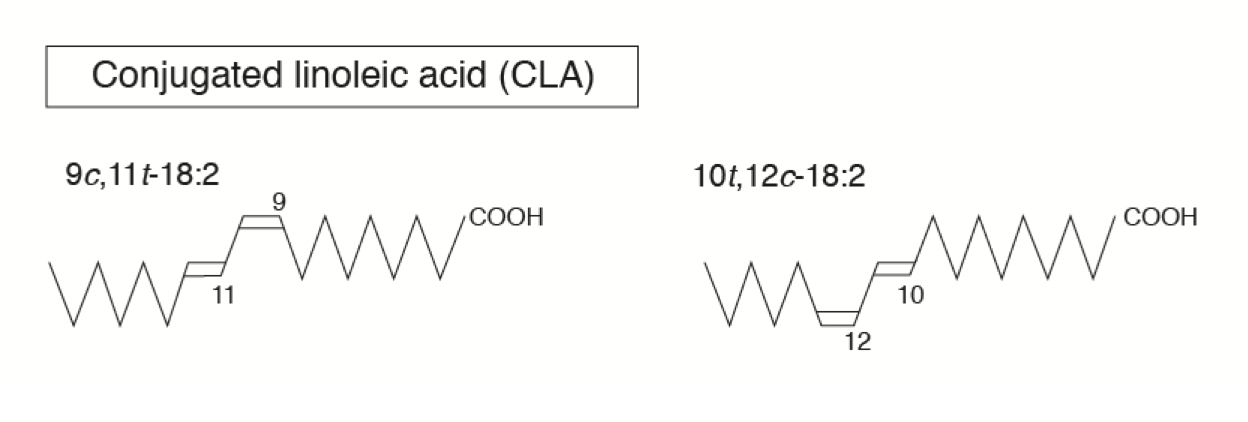
Cis versus Trans Fatty Acids
- Most natural fats occur in the cis form.
- The exception is a trans fat called conjugated linoleic acid (CLA; C18:2 n-6), which is produced by rumen microbes.
Cholesterol
Sterols (lipids with phenanthrene ring–like structures) are the most abundant steroid in the human diet. Cholesterol is the best known steroid (fat-soluble substance containing a steroid nucleus) and is the precursor of many other substances such as vitamin D, bile acids, sex hormones, and corticosteroid hormones.
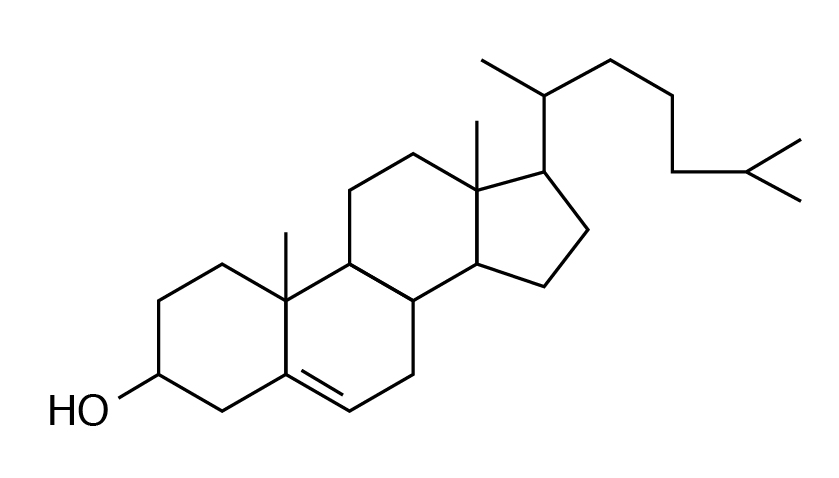 An important component of animal tissues, egg yolks, and cell membranes, cholesterol synthesis is partly by dietary intake and partly by biosynthesis from acetyl CoA. Excess cholesterol is stored in arteries and can lead to atherosclerotic plaque formation and cardiovascular disorders. Excretion of cholesterol is through bile acid formation. Plant cells do not contain cholesterol but instead contain other sterols called phytosterols.
An important component of animal tissues, egg yolks, and cell membranes, cholesterol synthesis is partly by dietary intake and partly by biosynthesis from acetyl CoA. Excess cholesterol is stored in arteries and can lead to atherosclerotic plaque formation and cardiovascular disorders. Excretion of cholesterol is through bile acid formation. Plant cells do not contain cholesterol but instead contain other sterols called phytosterols.
Key Points
- The lipid constituent of a feed is that portion that is soluble in organic solvents. Chemically, it is defined as an ester of fatty acids and glycerol. The most common form of lipids in plants is the triglyceride, but some parts of plants also contain compound lipids.
- Fats are made up of a glycerol backbone with fatty acids attached. We call these triglycerides, or more correctly, “triacylglycerol.”
- Triglycerides serve as energy reserves for the plant (seeds) or animal (fat depots).
- As the fat content of feed goes up so does its energy value.
- Functions of fats include providing energy, being components in the plasma membrane of all cells, being carriers for fat-soluble vitamins, and providing insulation and lubrication.
- Fatty acids may be saturated, unsaturated, or polyunsaturated. Palmitic and stearic acids are saturated, oleic acid is unsaturated, and linoleic acid is polyunsaturated.
- There are two essential fatty acids. These are linoleic (C18:2) and linolenic (C18:3).
- Arachidonic acid requirements may be met with linoleic acid (except in cats). Essential fatty acid can be omega-6 and omega-3 based on the position of the first double bond from the methyl (CH3) end.
- Conjugated linoleic acids (CLA) are a group of various fatty acid isomers synthesized by the rumen bacteria.
- CLA is 18 carbon atoms long with two double bonds separated by only one carbon, thus the name “conjugated.” Most recently, CLA has been discovered as a potent inhibitor of fat deposition. Other effects such as cancer prevention and promotion of immune health are also reported.
- Cholesterol is the most abundant steroid present in animal tissue and serves as a precursor for vitamin D, bile acids and steroid hormones.
- Cholesterol synthesis in the body is regulated by intake and by excretion through bile acid formation.
- Cholesterol deposits in arteries can lead to pathological disorders.
Review Questions
- What are the functions of lipids in animal diets?
- What is the difference between saturated, unsaturated, and polyunsaturated fatty acids?
- What is the difference between omega-3 and omega-6 fatty acids? Give an example of each.
- C20:5 n-3 is a fatty acid present in fish oil. Write three things about this fatty acid from its scientific notation.
- What is a conjugated fatty acid? Give an example.
- What is the difference between cis and trans fatty acids? Give an example of each.
- Why is it that we can pour salad dressing, while we need a knife to cut the fat around a steak?
- What are the essential fatty acids, and why are they essential?
- Which of the fatty acids are considered essential for cats?