III. Carbohydrates, Structures and Types
New Terms
Amylopectin
Amylose
Cellulose
Disaccharide
Fructose
Galactose
Glucose
Glycogen
Heteropolysaccharide
Homopolysaccharide
Monosaccharide
Oligosaccharide
Polysaccharide
Starch
Trisaccharide
Chapter Objectives
- To present the chemical structure of different types of carbohydrates and their importance in animal nutrition
Carbohydrates
What Are Carbohydrates?
Carbohydrates are the major components of plant tissue, making up to 60% to 90% of the dry matter (DM). Carbohydrates contain carbon, hydrogen, and oxygen in the proportion found in water (CH2O) and are hence hydrates of carbon. Carbohydrates are the basic energy source in animal cells. Dietary carbohydrates obtained from plant-based products serve as a major source of energy for the animal. The chlorophyll in plant cells traps solar energy and produces carbohydrates using carbon dioxide and water and gives off oxygen, as shown in the following equation:
solar energy + 6 CO2 + 6 H20 → C6H2O + 6 O2
In the plant cell, carbohydrates could be present in the cell content as sugar or starch, or they could be associated with the cell wall structure (e.g., cellulose). When animals eat plant materials (e.g., cereal grains, grass, fodder), energy in the feed’s carbohydrates is made available through metabolic processes in the animal cell. Overall, animal metabolism produces energy in a reverse process to that of photosynthesis.
Structure and Classification
One method of classifying carbohydrates is based on the number of carbon atoms per each molecule of a carbohydrate and on the number of molecules of sugar in the compound. Based on the number of carbon atoms, a carbohydrate can be classified as triose (3 C), tetrose (4 C), pentose (5 C), and hexose (6 C). The suffix “ose” at the end of a biochemical name flags the molecule as a “sugar.” Among these, pentoses (e.g., ribose in ribonucleic acid (RNA)) and hexoses (e.g., glucose, or blood sugar) are the most common sugars in animal tissues. Based on the number of molecules of sugar in the compound, carbohydrates can be classified as (1) monosaccharide, one unit of sugar; (2) disaccharide, two monosaccharides; (3) oligosaccharide, three to fifteen monosaccharides; and (4) polysaccharides, large polymers of simple sugars.
A. Monosaccharides are often referred to as simple sugars (e.g., glucose) and cannot be hydrolyzed into simpler compounds.
Monosaccharides can be subdivided based on the number of carbon (C) atoms. The following list shows the prefixes for numbers of carbons in a sugar.
- Triose (3 C)
- Tetrose (4 C)
- Pentose (5 C; e.g., Xylose and Ribose)
- Hexose (6 C; e.g., glucose, fructose, galactose, and mannose)
Most monosaccharides in animal tissues are of 5 C and 6 C sugars. Simple sugars are also subdivided into aldose, a sugar that contains an aldehyde structure, or ketose, a sugar that contains a ketone group. Both glucose and fructose have the same molecular formula C6H12O6 and are hexoses (6 C). But glucose is an aldose (also called aldohexose) and fructose is a ketose, or a ketohexose.
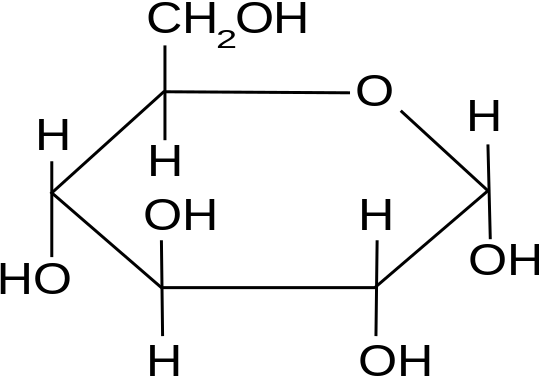
The three hexoses that are nutritionally and metabolically important are glucose, fructose, and galactose (see Figure 3.1).
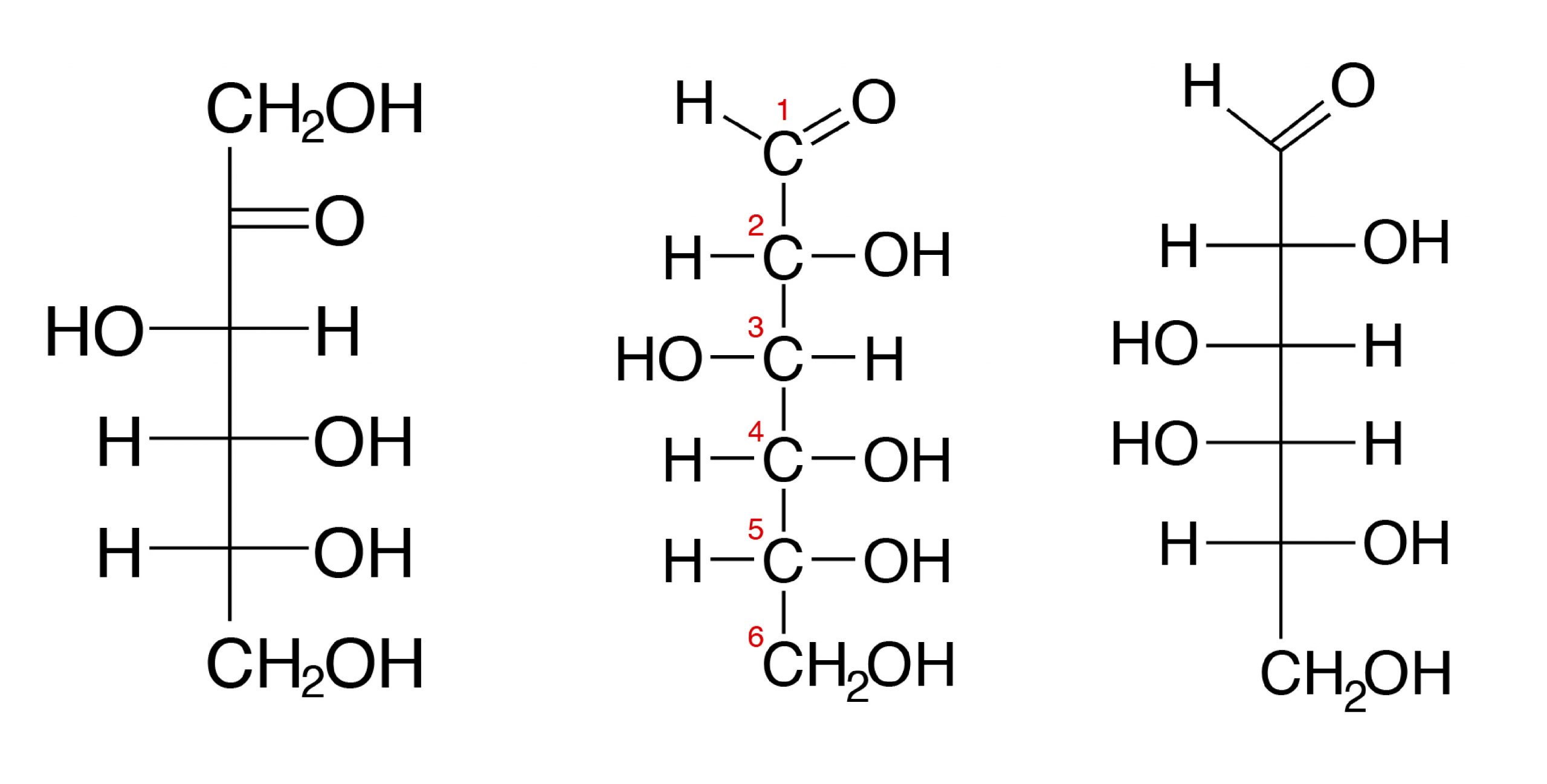
The chemical structure of glucose can be represented as a straight chain form (Figure 3.1) and in cyclic form (also shown in Figure 3.1). In a biological system, glucose exists primarily as a cyclic form and very rarely in a straight form (in aqueous solution). Glucose is the form of carbohydrates found in circulating blood (blood sugar) and is the primary carbohydrate used by the body for energy production. Fructose, or “fruit sugar,” is found in ripened fruits and honey and is also formed by digestion of disaccharide sucrose. Galactose is found along with disaccharide lactose in mammalian milk and is released during digestion.
Glucose can exist as α and β isomers and has immense animal nutritional implications. These two isomers differ in their orientation of OH on C #1.

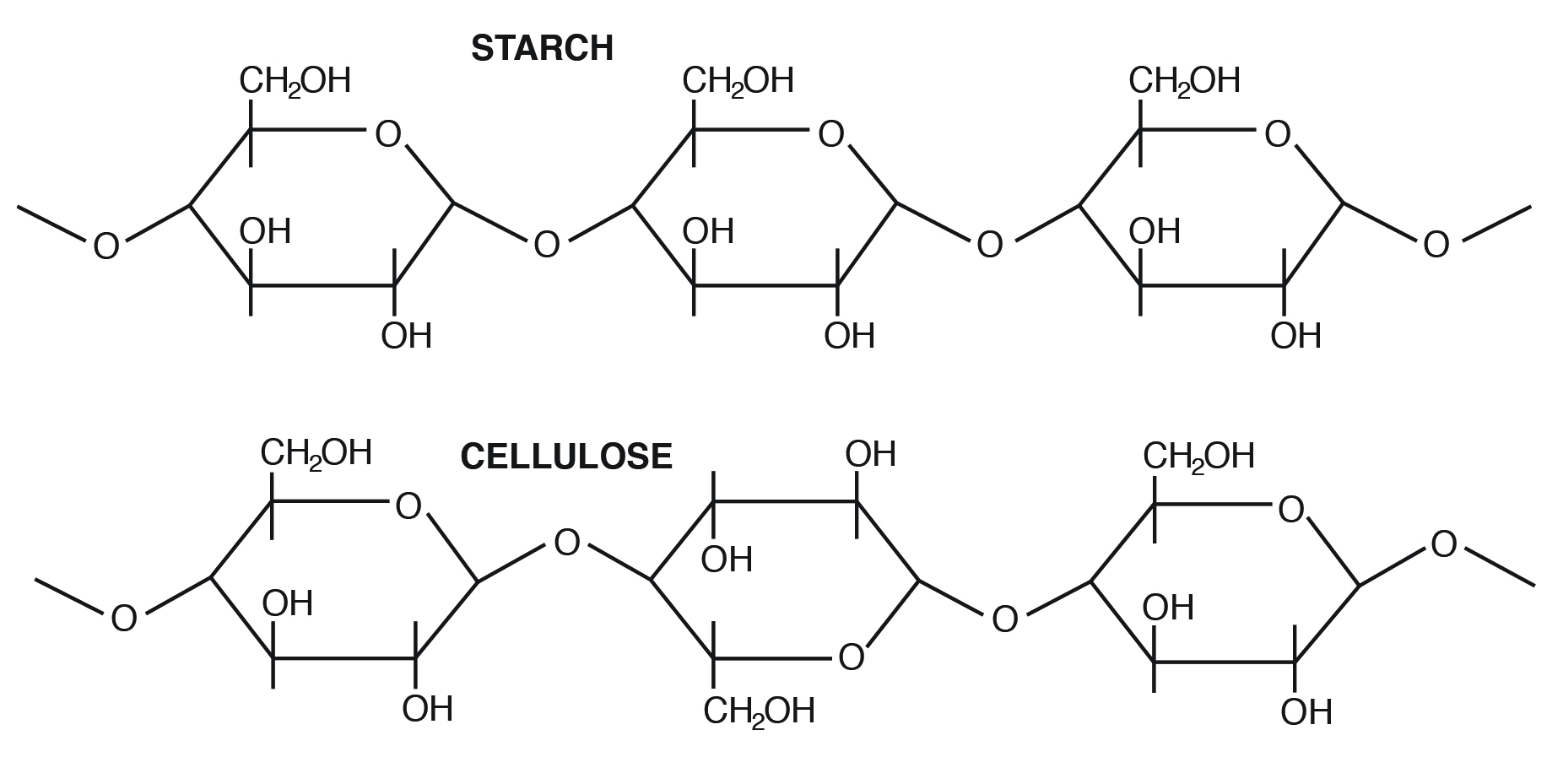
For example, starch contains α-D-Glucose, while cellulose has rigid polymers with β-D-Glucose. Nutritionally important sugars are of the D-form (not the L-form). D and L refer to stereo-orientation at asymmetric carbon position 5 in a hexose or carbon position 4 in a pentose.
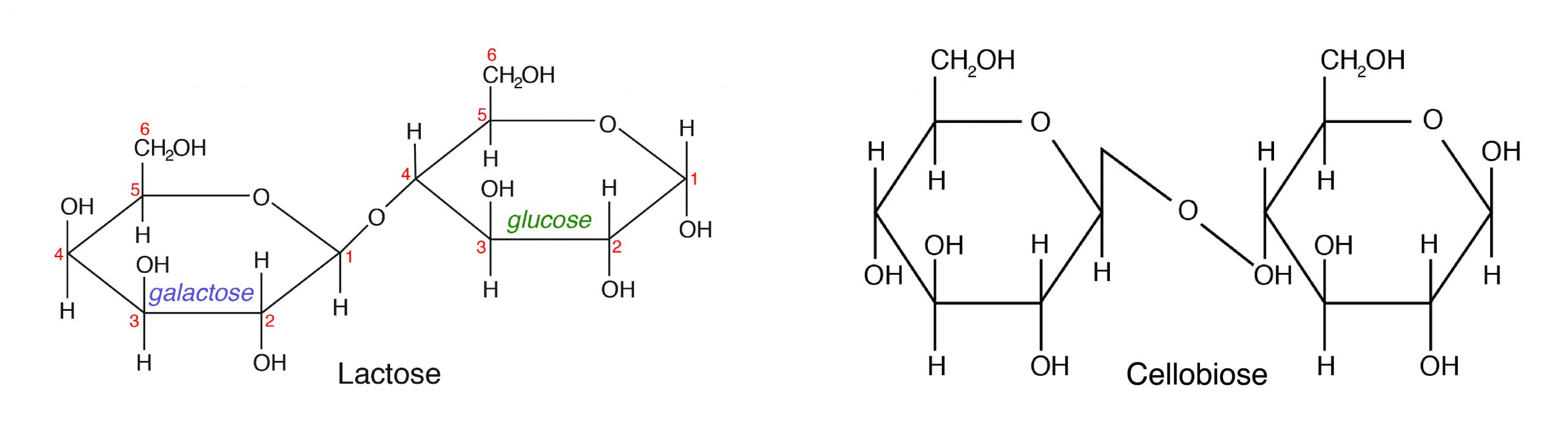
B. Disaccharides are made up of two monosaccharides bonded together by a glycosidic (covalent) bond. The following are some of the common disaccharides:
- Sucrose-glucose + fructose (e.g., table sugar)
- Lactose-glucose + galactose (milk sugar)
- Maltose-α-D-Glucose + β-D-Glucose (malt sugar)
- Cellobiose-β-D-Glucose + β-D-Glucose (cellulose)
Among the different disaccharides, lactose (milk sugar) is the only carbohydrate of animal origin. However, cellobiose as a component of cellulose is important in animal nutrition. Monogastric animals cannot digest cellulose because they do not produce the cellulase enzyme that can split β-D-Glucose.
C. Oligosaccharide are made by bonding together three or more (3 to 15) monosaccharides bonded together.
- Raffinose (glucose + fructose + galactose; 3 sugars)
- Stachyose (glucose + fructose + 2 galactose; 4 sugars)
In animal diets, oligosaccharides are commonly found in beans and legumes. Some oligosaccharides are used as substances to enhance the growth of good microbes (prebiotics). Recently, there has been an increased interest in the use of different oligosaccharides as feed additives to enhance hindgut health (e.g., fructooligosaccharides, mannan oligosaccharides).
D. Polysaccharides, as their name implies, are made by joining together large polymers of simple sugars.
Polysaccharides are the most important carbohydrate in animal feed. Polysaccharides are composed of many single monosaccharide units linked together in long, complex chains. The functions of polysaccharides include energy storage in plant cells (e.g., seed starch in cereal grains) and animal cells (e.g., glycogen) or structural support (plant fiber). Components of cell wall structure are also called nonstarch polysaccharides, or resistant starch, in animal nutrition, as they cannot be digested by animal enzymes but are fermented by hindgut and rumen microbes.
Polysaccharides can be homopolysaccharides or heteropolysaccharides.
- a. Homopolysaccharide
- b. Heteropolysaccharide
a. Homopolysaccharide: Contains only one type of saccharide unit.
Examples of homopolysaccharides that are important in animal nutrition include starch (nonstructural form), glycogen (animal form), and cellulose (plant structural form).
- Starch: Principal sugar form of carbohydrate in cereal grains (seed energy storage). The basic unit is α-D-Glucose. Forms of starch in cereal grains include
- Amylose-α 1,4 linkage-straight chain, nonbranching, helical structure
- Amylopectin-α 1,4 linkage with alpha 1,6 linkage at branch points
Amylose is the simplest of the polysaccharides, being comprised solely of glucose units joined in an alpha 1,4 linkage (Figure 3.4). Amylose is water soluble and constitutes 15% to 30% of total starch in most plants.
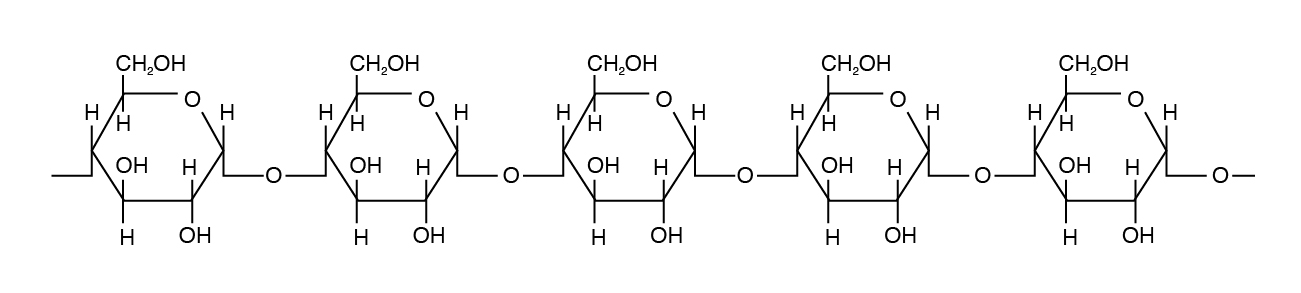
Amylopectin differs in how the glucose units are joined together. Alpha 1,4 linkages predominate, but a “branch” arises from an alpha 1,6 linkage. Such branches make the structure of amylopectin more complex than that of amylose. Amylopectin is not water soluble and constitutes 70% to 85% of total starch in plant cells.
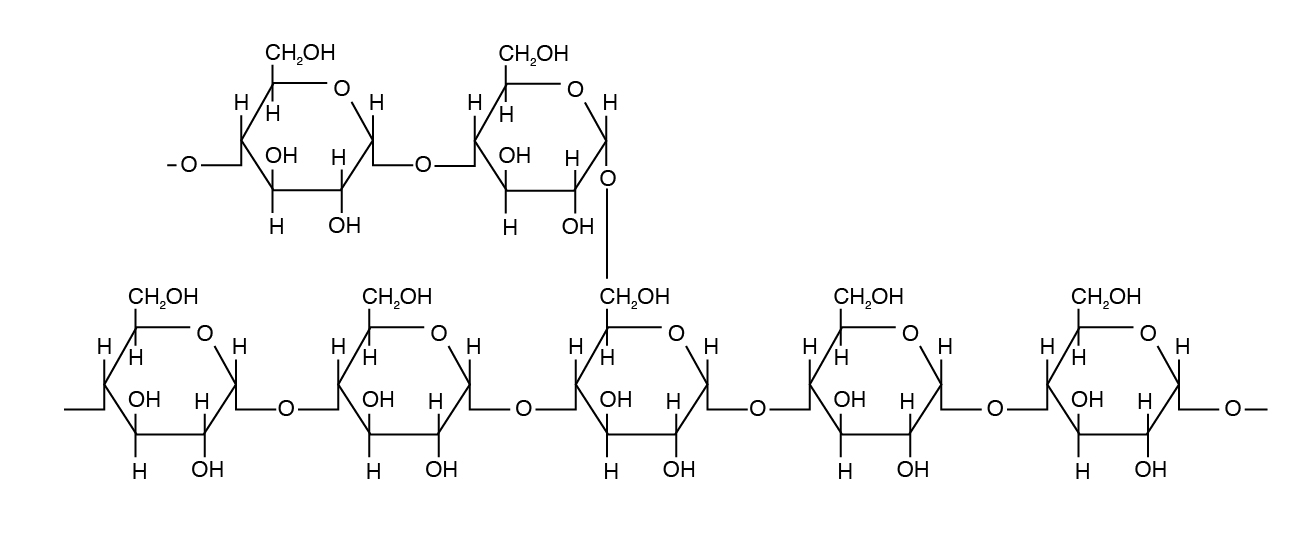
Glycogen is a form of starch found in animal tissue and is hence called animal starch. Glycogen is a polysaccharide that is physically related to amylopectin with basic alpha-D-Glucose but has a mix of α 1,4 and α 1,6 bonds. Glycogen exists in a small amount (< 1%) in liver and muscle tissue.
Cellulose is the most abundant carbohydrate in nature. It provides structural integrity to plant cell walls. The basic unit is β 1,4 linkage, straight chain, nonbranching (Figure 3.3). Cellulose is highly stable. No animal enzyme can break it; only microbial cellulase can degrade it. Ruminant animals such as cattle, however, have bacteria in their rumen that contain the enzyme cellulase. It breaks the beta 1,4 links of the glucoses in cellulose to release the sugar for energy.
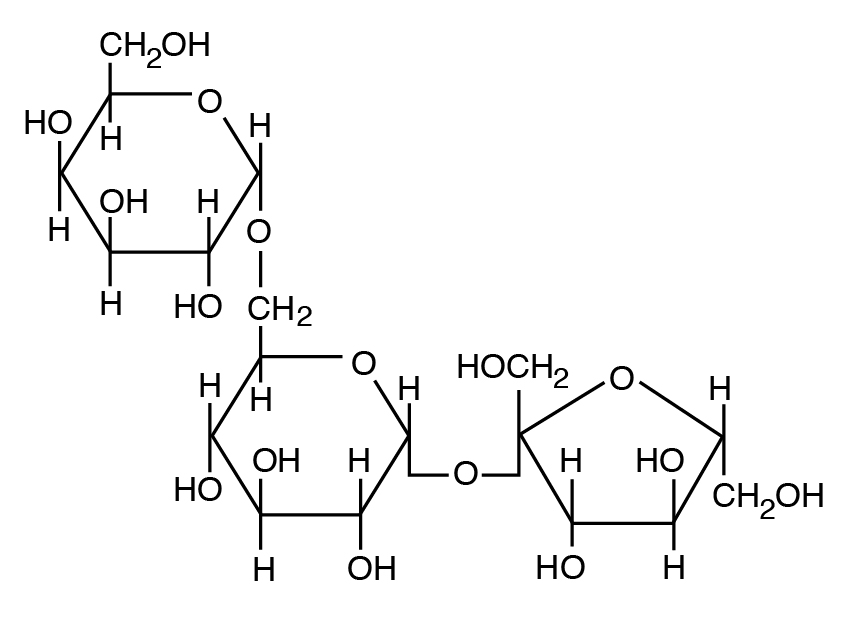
b: Heteropolysaccharide: A component of plant cell walls with a mix of 5 C and 6 C sugars (e.g., hemicellulose and pectin, a mixture of pentose and hexose units).
Key Points
- Carbohydrates are “hydrates of carbon” and have the generic structure of C(n)H(2n)O(n).
- A single sugar unit is a monosaccharide. These can consist of 3-carbon moieties (triose), 4-carbon units (tetrose), 5-carbon moieties (pentose), and 6-carbon moieties (hexose).
- Most nutritionally important sugars are pentoses or hexoses.
- Further classification of sugars is a definition of either aldose (having an aldehyde group) or ketose (having a ketone group). Glucose, mannose, and galactose are aldoses, whereas fructose is a ketose.
- Nutritionally important sugars are of the D-form (not the L-form). D and L refer to stereo-orientation at asymmetric carbon position 5 in a hexose or carbon position 4 in a pentose.
- Sugars link together via a glycosidic bond to form di- (two monosaccharides) or oligo- (3 to 15 monosaccharides), and polysaccharides.
- The nature of glycosidic bonds influences the structural and chemical properties of the sugars and influences their ease of digestion. Sugars that bond via an alpha 1,4 linkage may be digested by mammalian enzymes. Sugars that are linked via the beta 1,4 linkage are resistant to digestion.
- Nutritionally significant disaccharides are sucrose and lactose.
- Starch from plants serves as a major energy source in animal diets. Starch consists of two types of molecules: amylose (alpha 1,4 linked glucose) and amylopectin (alpha 1,4 and alpha 1,6 linked glucose).
- Glycogen, a storage form of carbohydrates in the liver and muscles, is very similar to starch also called animal starch.
- Plant polysaccharides also include cellulose and hemicellulose and pectin (nonstarch polysaccharides). Mammalian enzymes cannot degrade these polysaccharides to free sugars, but microbial enzymes can handle them.
Review Questions
- In what important ways do starch and cellulose differ?
- What are the disaccharides of nutritional significance?
- Nutritional important sugars are of the D-form or the L-form?
- The most important sugar in nutrition
- List the two forms in which starch exist
- The forms of starch in the animal body is?
- A structural homopolysaccharide made of glucose is
- cellulose
- hemicellulose
- pectin
- raffinose
- Among these different sugars, the primary source of energy for a broiler chicken is
- fructose
- sucrose
- glycogen
- glucose
- Two molecules of sugar are linked together by this bond
- peptic bond
- glycosidic bond
- diglyceride bond
- both a) and b)
- Among the two forms of starch, this is the major component of cereal grains
- amylose
- amylopectin
- cellulose
- glycogen