20 Microbial Symbioses
Symbiosis, strictly defined, refers to an intimate relationship between two organisms. Although many people use the term to describe a relationship beneficial to both participants, the term itself is not that specific. The relationship could be good, bad, or neutral for either partner. A mutualistic relationship is one in which both partners benefit, while a commensalistic relationship benefits one partner but not the other. In a pathogenic relationship, one partner benefits at the expense of the other. This chapter looks at a few examples of symbiosis, where microbes are one of the partners.
The Human Microbiome
The human microbiome describes the genes associated with all the microbes that live in and on a human. All 10^14 of them! The microbes are mostly bacteria but can include archaea, fungi, and eukartyotic microbes The locations include skin, upper respiratory tract, stomach, intestines, and urogenital tracts. Colonization occurs soon after birth, as infants acquire microbes from people, surfaces and objects that they come in contact with.
Gut microbes and human metabolism
Most of the microbes associated with the human body are found in the gut, particularly about 1-4 hours after eating a meal when the microbial population dramatically increases. The gut microbiota is extremely diverse and it has been estimated that from 500-1000 species of bacteria live in the human gastrointestinal tract (typically described as from 5-8 pounds of bacteria!).
The gut microbes are essential for host digestion and nutrition, aiding in digestion by breaking down carbohydrates that humans could not break down on their own, by liberating short chain fatty acids from indigestible dietary fibers. In addition, they produce vitamins such as biotin and vitamin K.
Gut microbes and obesity
There has been increased interest in the microbial gut population, due to the possibility that it might play a role in obesity. Although currently hypothetical, research has shown that obese mice have a microbial gut community that differs from the microbes found in the gut of non-obese mice, with more Firmicutes bacteria and methanogenic Archaea. It has been suggested that these microbes are more efficient at absorbing nutrients.
Human microbiome and disease
It has been shown that microbiota changes are associated with diseased states or dysbiosis. Preliminary research has shown that the microbiota might be associated rheumatoid arthritis, colorectal cancer, diabetes, in addition to obesity.
Research
The Human Microbiome Project (HMP) was an international research program based in the U.S. that was focused on the functions of gut microbiota. Some 200 researchers used advanced DNA-sequencing techniques to determine what microbes are present and in what populations.
Many current research projects are focused on determining the role of the human microbiome in both health and the diseased state. There is no doubt that our knowledge will continue to grow as we find out more about the vast populations of microbes that live in and on us.
Biofilms
Biofilms are a complex aggregation of cells that are encased within an excellular matrix and attached to a surface. Bioforms can form on just about any surface and are common in nature and industry, being found on the surfaces of rocks, caves, pipes, boat hulls, cooking vessels, and medical implants, just to name a few. They have also been around a long time, since the fossil record shows evidence for biofilms going back 3.4 billion years!
The microbial community of a biofilm can be composed of one or two species but more commonly contains many different species of bacteria, each influencing the others gene expression and growth.
Biofilm development
The basic steps for biofilm formation can be broken down into four steps:
- Cell disposition and attachment – in order for biofilm development to occur, free-floating or planktonic cells must collide with a suitable surface. Typically the surface has been preconditioned with the deposits of environmental proteins and other molecules.
- Colonization – cell-to-cell signaling occurs, leading to the expression of biofilm specific genes. These genes are associated with the communal production of extracellular polymeric substances. DNA released by some cells can be taken up by others, stimulating the expression of new genes.
- Maturation – the EPS matrix fully encases all the cells, as the biofilm continues to thicken and grow, forming a complex, dynamic community. Water channels form throughout the structure.
- Detachment and sloughing – individual cells or pieces of the biofilm are released to the environment, as a form of active dispersal. This release can be trigger by environmental factors, such as the concentration of nutrients or oxygen.
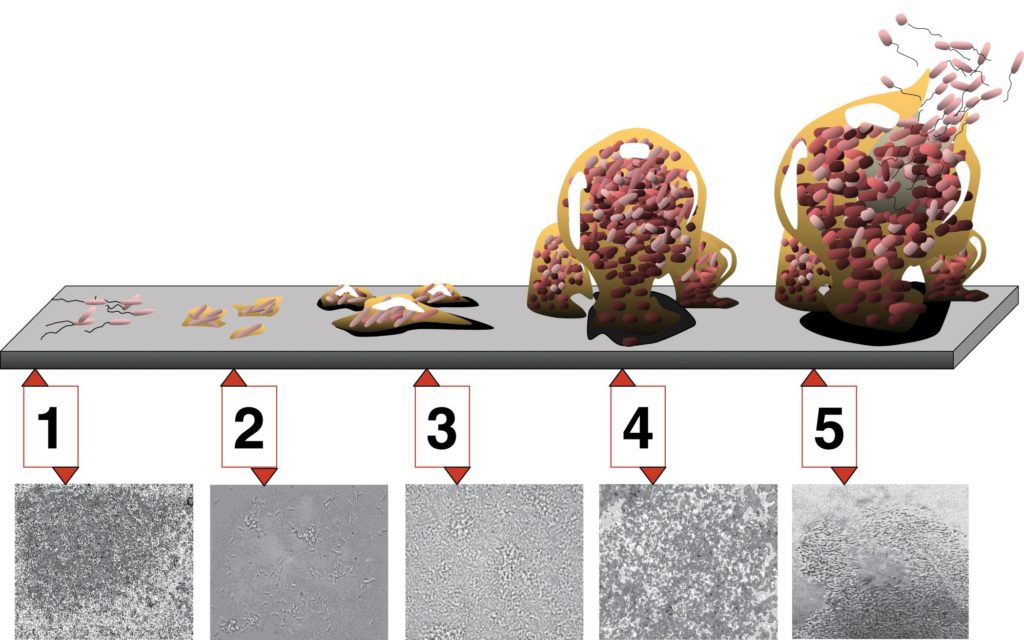
Biofilm Development. Each stage of development in the diagram is paired with a photomicrograph of a developing Pseudomonas aeruginosa biofilm. All photomicrographs are shown to same scale. By D. Davis [CC BY 2.5], via Wikimedia Commons
Cellular advantages of biofilms
Why do bioforms develop? There are certain advantages that cells enjoy while in a biofilm, over their planktonic growth. Perhaps most importantly, biofilms offer cells increased protection from harmful conditions or substances, such as UV light, physical agitation, antimicrobial agents, and phagocytosis. It has been shown that bacteria within a biofilm are up to a thousand times more resistant to antibiotics than free-floating cells!
A biofilm also allows a cell population to “put down roots,” so to speak, so that they can stay in close proximity to a nutrient-rich area. For example, a biofilm that develops on a conduit pipe at a dairy plant will have continual access to fresh food, which is much better than being swept away with the final product.
Lastly, biofilms allow for cells to grow in microbial populations, where they can easily benefit from cell-to-cell communication and genetic exchange.
Biofilm impacts
Biofilms have huge impacts throughout many different types of industry. Medical implants ranging from catheters to artificial joints are particularly susceptible to biofilm formation, leading to huge problems for the medical industry. Biofilms are responsible for many chronic infections, due to their increased resistance to antimicrobial compounds and antibiotics. A type of biofilm that affects almost everyone is the formation of dental plaque, which can lead to cavity formation.
Outside of medicine, biofilms affect just about any industry relying on pipes to convey water, food, oil, or other liquids, where their resistance makes its particularly difficult to completely eliminate the biofilm.
Quorum Sensing
The word quorum refers to having a minimum number of members needed for an organization to conduct business, such as hold a vote. Quorum sensing refers to the ability of some bacteria to communicate in a density-dependent fashion, allowing them to delay the activation of specific genes until it is the most advantageous for the population.
Quorum sensing involves cell-to-cell communication, using small diffusible substances known as autoinducers. An autoinducer is produced by a cell, diffusing across the plasma membrane to be released into the environment. As the cell population increases in the environment the concentration of autoinducer increases as well, causing the molecule to bind to specific cellular receptors once a threshold concentration has been reached. The autoinducer then diffuses into the cell, often binding to a specific transcription factor. This produces a conformational change that allows the transcription factor to bind to the cell’s DNA, triggering expression of specific genes.
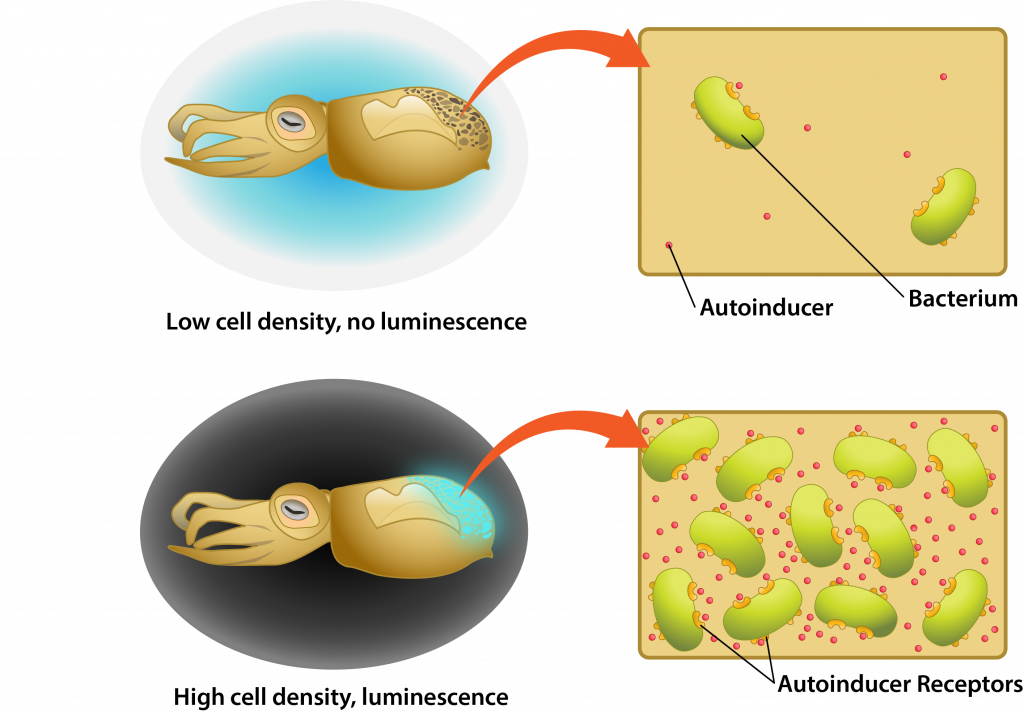
Quorum sensing example
One of the best studied examples of quorum sensing is the mutualistic relationship between the bioluminescent bacterium Aliivibrio fischeri and the bobtail squid. The bobtail squid actually has a light organ that evolved to house the bacterium, relying on its luminescence to provide a camouflage effect against predators. At low cell density the luminescence would not provide the desired effect, representing a waste in energy by the bacterial population. Therefore, quorum sensing is used so that the lux gene that codes for the luciferase enzyme necessary for luminescence is only activated when the bacterial population is at sufficient density.
Key Words
symbiosis, mutualistic, commensalistic, pathogenic relationship, human microbiome, dysbiosis, Human Microbiome Project (HMP), biofilm, planktonic, extracellular polymeric substances/EPS, quorum sensing, autoinducer.
Study Questions
- What is a symbiosis? What are different specific examples of symbioses?
- How is the human microbiome defined? What microbes does it include?
- What impact does or can the human microbiome have on its host?
- What are biofilms? Where do they form?
- What are the steps leading to biofilm formation?
- How do cells benefit from existing in a biofilm? What are the impacts of biofilms to humans?
- What is quorum sensing? How does the mechanism work?
- Describe the example of Aliivibrio fischeri and the bobtail squid. What role does quorum sensing play in the relationship between these two organisms?

