8 The Cell Cycle and Mitosis
Introduction
For most of this textbook, we have looked at each structure and process separately so that we can better understand them. We have focused on how the underlying chemistry of structures is essential to understanding the function of each organelle and each individual process. Here we are finally able to integrate all of the different bits of the cell and see how it all comes together as a whole and drives complex cellular processes. More specifically, we will be exploring the process of cell growth and division. We assume that this is not your first exposure to the concepts of cell growth and division (also known as mitosis and cytokinesis), and we approach the topic from that lens. If you need a refresher on the basics, we encourage you to return to the introduction and explore the review material.
Cell growth and division are moments within a larger process known as the cell cycle. The cell cycle impacts every aspect of cellular function. Every organelle gets involved in some way or another at every step of the way. Growth requires protein and membrane synthesis, energy production, transport of materials, and more. Selecting the correct moment to undergo cell division requires careful coordination of both the internal and external environment of the cell. Cells that divide at the wrong moment are unlikely to survive the process, so it must be carefully timed and controlled. Mitosis itself requires a complete and total disruption of cellular function as well as a complete but temporary rearrangement of the cell’s contents. This, too, requires precise coordination of the organelles. In this, the final chapter of this textbook, we will explore how the cell coordinates all of the various processes we’ve learned about so far in order to grow and then, at just the right moment, divide into two daughter cells.
Topic 8.1: Regulating the Cell Cycle: Checkpoint Control
Learning Goals
- Review the stages of the cell cycle, including the checkpoints, and identify the key features of each stage.
- Describe how specific protein modifications (e.g., phosphorylation and ubiquitination) result in activation/deactivation of cyclin-CDK complexes to regulate cell cycle checkpoints.
- Explain how the activation of the cyclin-CDK complexes results in the start of the next phase of the cell cycle. Use examples from both M- and S-cyclin-CDK complexes to explain this.
- Detail how fluorescence-activated cell sorting (FACS) can be used to identify the stage of the cell cycle for a population of cells.
Introduction to the Cell Cycle and Checkpoints
A discussion of the cell cycle and mitosis is a very good way to end this book, as it is a wonderful example of how the concepts we’ve covered in this book are interconnected. The progression of the cell from interphase to cell division is precisely regulated, and it involves every other cellular component in some way.
The cell cycle is defined as the events that enable cells to proceed from one cell division event to the next. Cell division itself consists of the overlapping processes of mitosis (nuclear division) and cytokinesis (division of the cytoplasm).
The cell cycle is divided up into four separate phases based on the primary event that is taking place in that stage:
- G1 (gap or growth 1) phase: This is the “gap” between the end of cytokinesis and the start of DNA synthesis. A lot of the work of this phase involves cell growth so that it can support itself and also have the resources it needs for the next phase.
- S (synthesis) phase: This phase is defined by the initiation and termination of DNA synthesis.
- G2 (gap or growth 2) phase: This second “gap” phase lasts from the end of DNA synthesis to the onset of mitosis. The cell continues to grow but also prepares for what’s to come in the next phase.
- M (mitosis) phase: This is the phase in which cell division occurs.
Figure 08-01 shows an overview of the stages of the cell cycle. Collectively, we consider G1, S, and G2 to be interphase (i.e., the phases “in between” M phase).
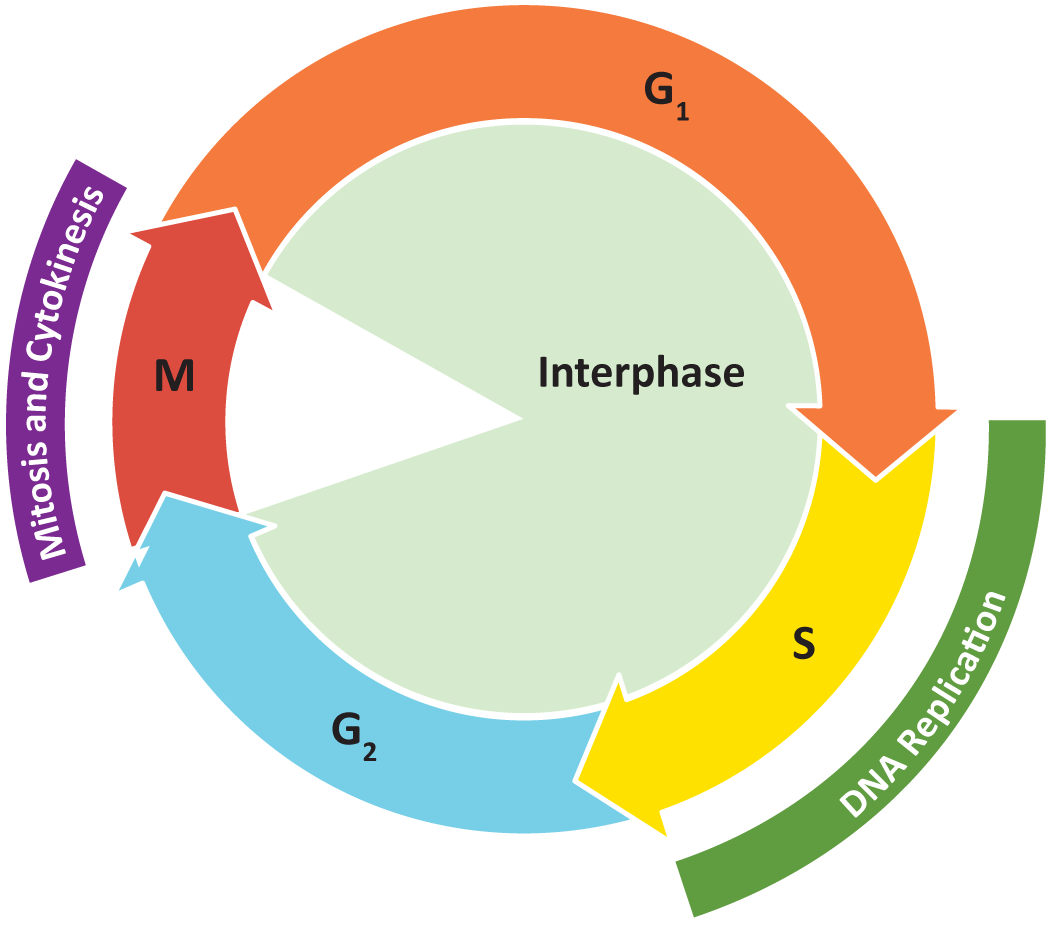
It is important to keep in mind that while cells do need a mechanism to control growth, that is not always their primary concern. There must also be provision for cells to “step away” from the cell cycle for a time and into other developmental pathways (mating, meiosis, differentiation). Some cell types will hit a point in their development where division isn’t a good option anymore for one reason or another, so they withdraw from the continuous cycle of growth and replication. Whether the cell leaves the cell cycle on a temporary or permanent basis is also highly controlled, and we will discuss how the cell makes these kinds of decisions later in this topic. (Hint: Signaling proteins are involved.)
Signaling and regulation are at the heart of the proper progression of the cell cycle. Using these tools, the cell ensures that
- discrete events, such as DNA synthesis and mitosis, do not occur before the cell is ready, and they occur in the right order
- the cell pauses DNA replication or mitosis when errors are identified and repairs are attempted
- only cells that should divide are allowed to do so
- Some cells must undergo terminal differentiation, stall temporarily or permanently, or even go through programmed cell death (i.e., apoptosis). These are all cell cycle decisions.
- growth is coordinated with cell division so that the size of cells is maintained over the many cellular generations
- Some cells are designed to get bigger or smaller over several cell cycles. Some even divide asymmetrically so that one large and one small daughter cell is produced. All of these will require precise cell cycle control.
One of the most important controls placed on the progression of the cell cycle is a series of checkpoints in which the cell is required to meet certain criteria before it is allowed to proceed. In this way, these checkpoints act as a form of quality control. The control of the checkpoints and the mechanism of when/how cell division takes place is a perfect example of how the cell uses signaling to understand its environment and to effect internal change. Cells do not pass through these checkpoints randomly. They are constantly receiving cues from the exterior (such as growth factors, for example) and from the interior that help them decide exactly when and how to divide.
Each of these checkpoints is controlled by one or more “gatekeeper proteins” that respond to cellular conditions and will only allow the cell to move forward into the next phase of the cell cycle if conditions are “right.” As a result, the checkpoints help ensure that specific criteria are met before the cell cycle is allowed to continue. There are a few different checkpoints, but the ones we are going to focus on are the following: the G1/S checkpoint, which allows the cell to pass into S phase, and the G2/M checkpoint, which controls when the cell enters mitosis. We will also look at the checkpoint that is in the middle of mitosis, which ensures that the mitotic spindle is set up correctly prior to chromosome separation. We call this the metaphase checkpoint, but we have seen many other names used as well (spindle assembly checkpoint, M phase checkpoint, M/G1 checkpoint, etc.).
Cells can only move through the checkpoint and into the next stage of the cell cycle when they have met the required conditions. For example, if biotin, a vitamin, is missing from the growth medium, yeast cells will not pass the G1/S checkpoint even if all other conditions are perfect. Low nutrient levels reduce the growth rate, which, if severe enough, can make it so a newly divided cell will not survive. Thus, having the ability to confirm that everything is in place before dividing is key to survival for the cell.
G1/S Checkpoint
The first checkpoint a new cell will encounter is the G1/S checkpoint (also sometimes known as the “restriction” point or “Start”). As its name implies, this checkpoint marks the transition from G1 to S phase. Since S phase involves the replication of DNA, it is important that the cell and the environment are both ready before replication starts. Not only is this an energy- and nutrient-intensive process, but replication is when any preexisting errors in DNA become permanent mutations, so the DNA must be in good shape before the cell starts this process. Once again, making an error with the timing of replication could result in the death of the cell, so the checkpoint plays a key role here.
Cell signaling is important for ensuring that conditions are ideal for cell division. The cell responds to internal and external cues in order to “decide” when to divide. Some of the conditions that must be met include the following:
- Proper nutrients (carbon source, energy source, inorganic phosphate, nitrogen, vitamins, etc.) must be present at specific concentrations.
- Sister chromatid separation (from the previous mitosis) must be complete.
- There must be no detectable DNA damage.
- The cell must have reached a critical threshold size.
Additionally, external factors must also be appropriate. For example, in yeast, if the appropriate mating factor is present in the environment, the cells cannot proceed to S phase and are switched instead into an alternative pathway (called the sexual pathway). Similarly, in mammalian cells, appropriate growth factors must be present to allow cells to pass this checkpoint. If not, the cell remains in G1.
In some cases, cells are stalled for extended periods of time…maybe indefinitely. We say that these cells have removed themselves from the cell cycle and that they are in G0 phase. This could be due to a long-term deprivation of nutrients or other resources required for cell division. More commonly, however, this is a normal part of the development of certain cell types. For example,
- Stem cells for specific tissues will enter G0 for short periods of time until replacement cells are required. This allows tissues to grow to a certain size and then stop growing and maintain a relatively stable size and distribution. Your blood cell system (called the hematopoietic system) does this. New blood cells are grown only when specific cell types are needed or cells are lost through injury.
- Some cell types undergo terminal differentiation, which means that when these cells reach maturity, they do not need to go through mitosis anymore. Good examples of this are muscle cells (multiple muscle cells fuse together at maturity to produce multinucleate muscle fibers), neurons (with their extremely long axons), and osteocytes (bone cells, which are intricately embedded in the calcified matrix of the bone).
G2/M Checkpoint
The second is the G2/M checkpoint, which stops the cell from entering mitosis before its ready. It is also sometimes called “CD” or “Commitment to Division,” as the cell cannot stop the process of mitosis once it has passed this point.
As its name suggests, this checkpoint controls the transition from G2 to M phase. Some conditions that must be met here are the following:
- DNA replication must be complete and accurate. No DNA damage can be detected (through a robust biochemical surveillance system) or this checkpoint cannot be passed. This is the most important factor for passing the G2/M checkpoint.
- The cell must also have reached a certain minimum size so that it is big enough that, when split in two, the two daughter cells will also be large enough to survive.
It is considered exceedingly rare that cells would stall and enter G0 from G2. One would think that there is not really much point in doing all of the work of replication unless there is an intention for the cell to complete mitosis. However, since this is biology, there are examples of chromosomes called polytene chromosomes that can have thousands of sister chromatids, instead of simply two. These cells undergo repeated rounds of replication without moving forward to mitosis. It is thought to help increase the number of copies of genes in the cell, which can significantly impact gene expression. While this is thought to be relatively common in some cell types, such as the salivary glands of certain insects (like flies), and examples can be found throughout eukaryotes, it is still considered to be a relatively rare occurrence overall.
Metaphase Checkpoint
This checkpoint marks the halfway point of mitosis, but it’s also the point right before the actual division of the genetic material, so it makes sense that this would be a point that the cell would verify before proceeding. To separate the sister chromatids of the chromosome, the mitotic spindle, composed of microtubules and associated motor proteins, must be assembled, and the chromosomes must be properly attached. If the spindle is not assembled correctly, an entire chromosome could get destroyed or mislocalized. Considering how important the genetic material is to the cell, you can imagine how bad this kind of “mitotic misfire” would be; both daughter cells would likely die, if they could even complete mitosis. Interestingly, you can see this checkpoint if you watch closely as cells divide under the microscope (Video 08-01). The chromosomes line up at the metaphase plate and then wait at the checkpoint for a little while until suddenly the sister chromatids split and move to opposite ends of the spindle.
Cell Cycle Checkpoint Control: Cyclins and CDKs
Like all things in cell biology, there was a time when the details of the cell cycle were not known to scientists. Mitosis was observed extremely early (as early as the 1600s), as even the most rudimentary light microscopes could be used to observe it. The fact that cells arose from other cells was identified in the 1800s, but it wasn’t until the 1950s that the rest of the cell cycle was suggested. By the late 1960s, scientists believed that “something” in the cytoplasm was controlling the cell cycle, but they had no proof. Dr. Yoshio Masui, a Japanese Canadian researcher at the University of Toronto, ran some experiments while working as a postdoctoral fellow at Yale University that provided the evidence needed. In these experiments, Dr. Masui extracted cytosol from frog’s eggs that were in mitosis and then injected it into a cell that was stalled at the end of interphase. (Aside: This is a normal part of frog oocyte maturation.) As a control, they compared this with oocytes that were injected with cytosol from another oocyte also in interphase. They found that when the cytosol from a mitotic oocyte was injected into the interphase oocyte, a mitotic spindle would begin to form. This did not happen in the control, in which interphase cytosol was injected into an interphase oocyte.
The results of these experiments provided evidence of the following:
- The active agent that promotes the next stage of the cell cycle is in the cytosol.
- Control of DNA synthesis and mitosis is positive—that is, the active agent promotes mitosis in the recipient cell.
- Cells can be advanced into the next stage before they planned it by adding the appropriate factors to their cytosol.
After this initial discovery, it was replicated using cytosol from mitotic cells from many different species and by taking cytosol from one species and using it to induce mitosis in another species. This meant that the “factor” in the cytosol was universal and helped promote the “maturation” of the cell. As a result, they called this molecule the maturation promoting factor (MPF). It was called a factor, and not a protein, because at this point, no one knew what it was. About 20 more years of research were required to figure out that the “factor” in question was actually a protein.
MPF = Activated Cyclin-CDK Complex
We now know that MPF is actually a set of proteins that work together to control the checkpoints and thus the entire cell cycle. The active agent is a protein called cyclin-dependent kinase (CDK). As you remember from Chapter 7, a kinase is an enzyme that specifically adds phosphate groups to other proteins. This particular kinase is only active in the presence of a second protein known as cyclin. Cyclin binds to CDK (Figure 08-02), and acts as a regulatory unit; CDK can only perform its function as a kinase when cyclin is bound. If cyclin is removed from CDK, then CDK is inactivated. This is also the source of its name…cyclin-dependent kinase. These two proteins combine to produce a single enzyme called the cyclin-CDK complex. The cyclin-CDK system is somewhat unique due to the use of cyclins to regulate CDK activity. The cell controls the activity of CDKs by controlling the synthesis and destruction of cyclins.
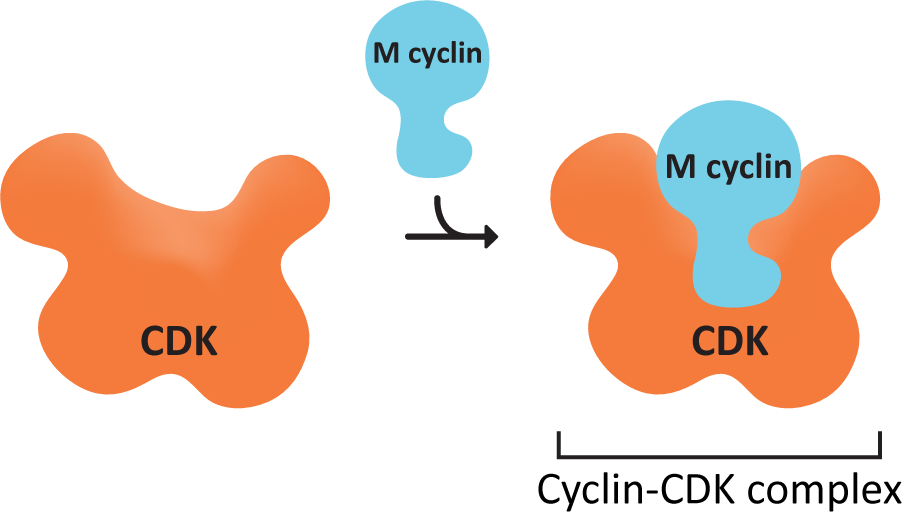
CDK concentrations in the cell remain constant throughout the cell cycle, but they are not always active. On the other hand, cyclin concentrations show a cyclical pattern. They increase as the cell moves through the cell cycle, which coincides with increasing enzymatic activity of CDK, peaking at the appropriate point in the cell cycle (usually a checkpoint). After the relevant checkpoint is passed, cyclin concentrations crash down to almost nothing. Once again, this change in cyclin concentration coincides with the end of the CDK enzymatic activity. It is this “cycling” of the cyclin concentrations that regulates CDK activity, allowing the cell to progress through checkpoints of the cell cycle.
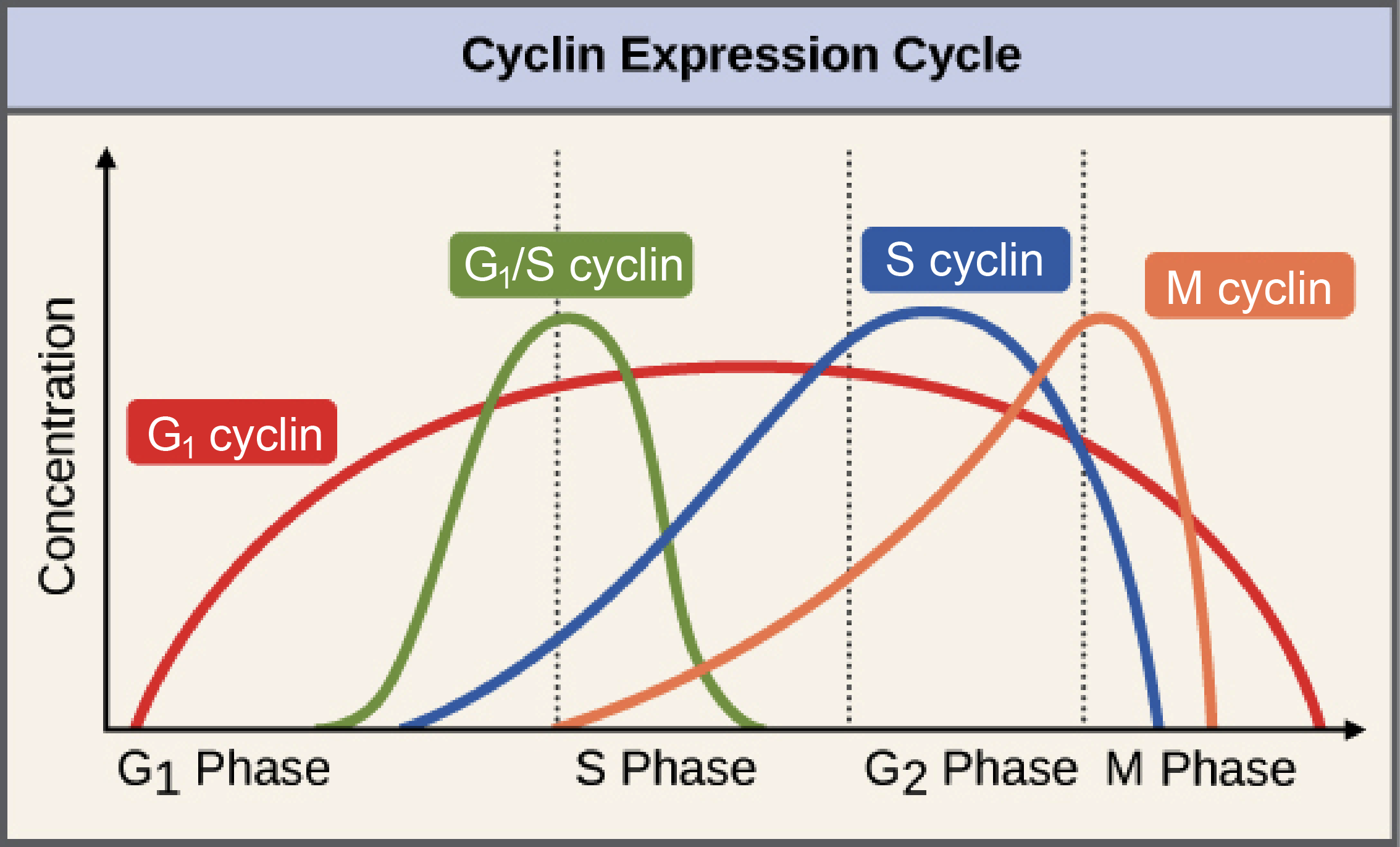
Since the initial studies last century, we have learned that there are actually several classes of cyclins and CDKs, and each of these classes is responsible for controlling a specific part of the cell cycle. The four major classes of cyclins are listed in Table 08-01.
| Mitotic Cyclin Classes | |
|---|---|
| Cyclin class | Function |
| G1 cyclins | Unique cyclins that are thought to help the cell respond to external signals to leave G0 and initiate cell division. We won’t discuss these any further in this book. |
| G1/S cyclins | Cyclins that control the G1/S checkpoint and control the transition from G1 to S phase. |
| S cyclins | Cyclins that activate at the start of S phase (by the G1/S cyclins) and directly induce replication of DNA. The concentration of these cyclins remains high right through to M phase. |
| M cyclins | Cyclins that control the G2/M checkpoint. They remain active in the first half of mitosis until their destruction is signaled by the anaphase-promoting complex (APC). |
Increasing cyclin concentrations occur directly prior to a cell passing a cell cycle checkpoint. This is because the cyclin-CDK activity must hit a certain level before the checkpoint can be passed, and cyclins are required components of CDK activity. In some cases, such as the M cyclin shown in Figure 08-03, the activity of the CDK decreases rapidly not long after the checkpoint has been passed, but in others, like the G1-cyclin shown here, the activity of the CDK is activated at one checkpoint and then stays high throughout the rest of the cycle.
Phosphorylation Controls Cyclin-CDK Enzymatic Activity
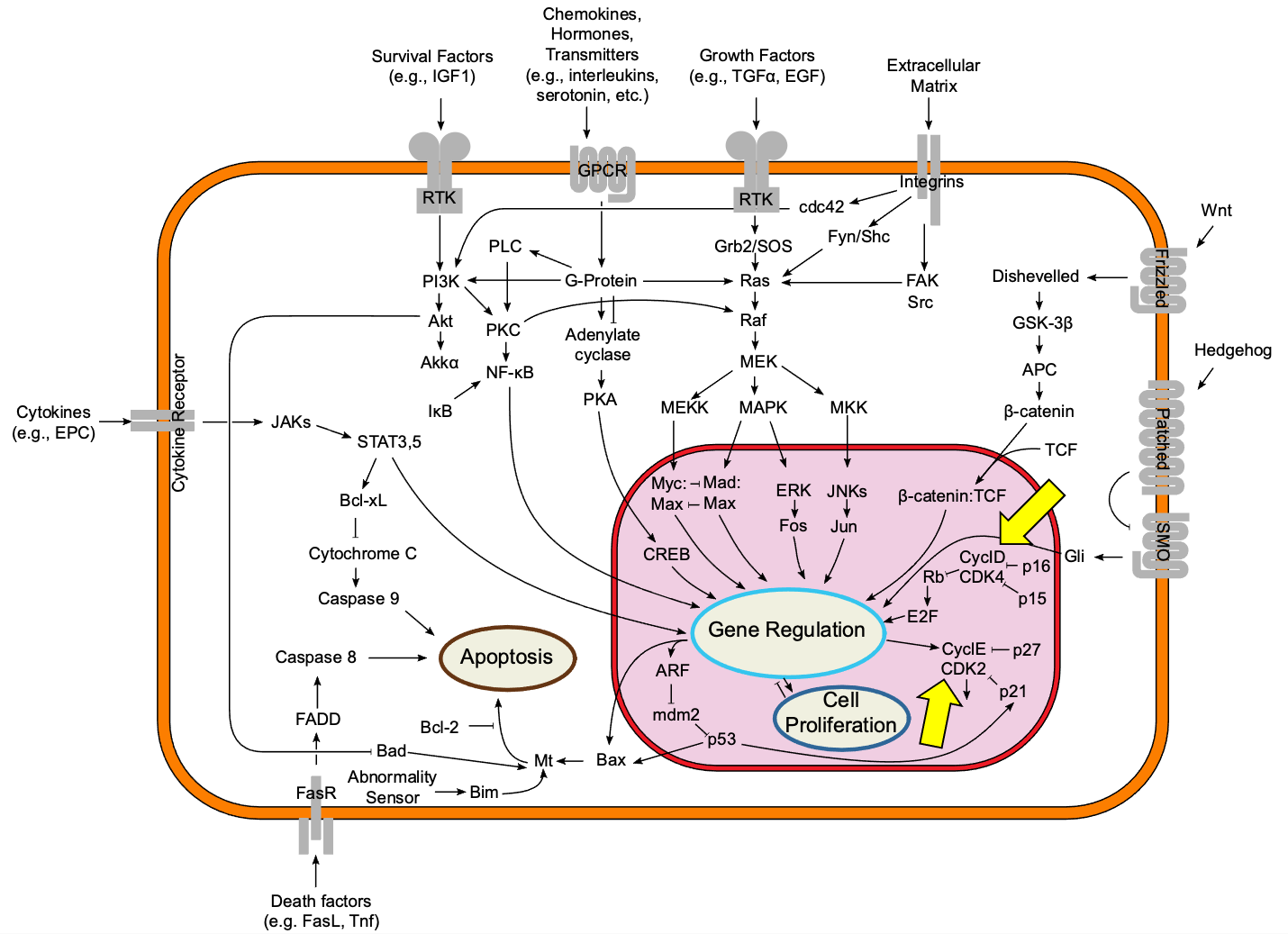
The activity of the CDKs must be very tightly controlled. The consequences of a cell moving to the next stage of the cell cycle before its ready could be disastrous. As such, cyclin-CDKs are at the heart of a complex signaling pathway involving potentially hundreds of enzymes that are fighting with each other to either activate or deactivate the cyclin-CDK complex. Figure 08-04 shows an extremely simplified version of a regulatory pathway that includes two different cyclin-CDKs (highlighted by yellow arrows). In particular, take note of how the pathways labeled cell growth (labeled as cell proliferation in the figure) and programmed cell death (also known as apoptosis) are connected to each other. This gives a strong hint about how important it is to get this right. If it goes wrong, the cell has the option to initiate apoptosis and die if necessary.
Because CDK activation must be tightly controlled, there are multiple layers of regulation. Note that while the binding of cyclin to CDK is necessary for CDK activity, it is not sufficient for activation of the CDK on its own. (Remember our discussion of necessary and sufficient from Chapter 3.) This means that other factors are also needed to activate the CDK complex. In this case, the CDK-cyclin complex itself must also be phosphorylated (by other kinases). Interestingly, phosphorylation of CDK can also be used to keep the CDK inactive as well, depending on the location of the phosphate addition on the protein.
The activation of CDKs follows a set pattern, which requires both the presence of cyclin and proper phosphorylation of the CDK. We’ll use the activation of the M-CDK/cyclin complex as an example, which is illustrated by Figure 08-05 and Video 08-02. In a nutshell, the binding of cyclin initiates the activation process. The signaling proteins upstream activate two kinases in particular: Wee1 and CAK. Both phosphorylate the CDK, but for different purposes. The phosphate group that Wee1 adds is considered to be an inhibitory phosphate, which limits the activity of the CDK. On the other hand, CAK (which stands for cyclin-activating kinase) adds a phosphate that is necessary for the CDK to become active. Once everything else is ready and it’s time for the checkpoint to be passed, a third protein called cdc25 comes in and removes the inhibitory phosphate (thus cdc25 is a phosphatase), and the CDK becomes fully active.
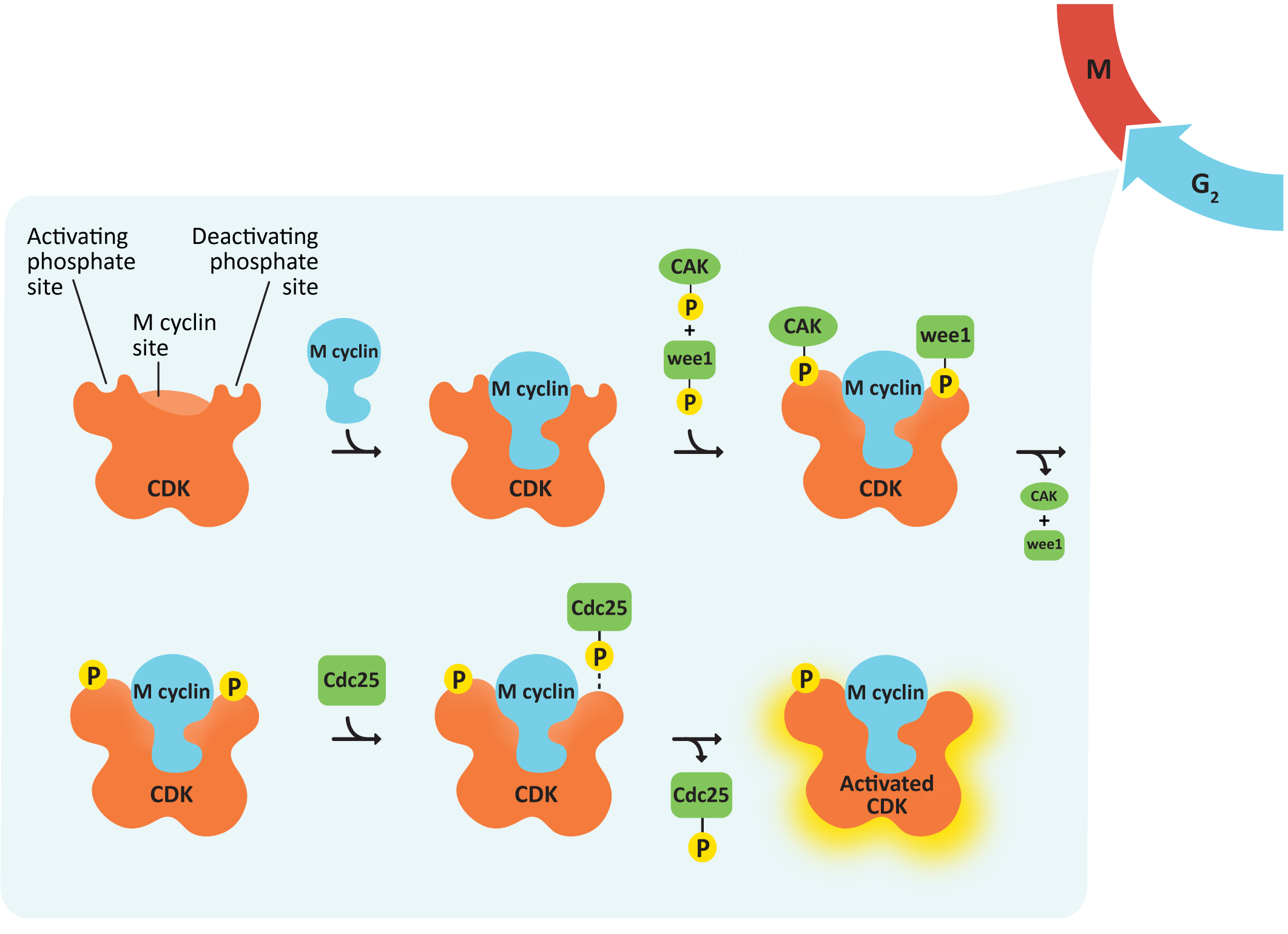
You can think about this a little bit like a race car at the starting line of a race. By the time you get to the starting line, the car is all gassed up (cyclin) and running (activating phosphate). The drivers might even be revving the engines a bit, but with the break on (inhibitory phosphate) so that the car can’t go anywhere until the signal is received that the race has started. By being ready to go before the race starts, they can leave the starting line as quickly as possible once the signal to start is received.
In yeast, Wee1 and Cdc25 are key regulators of M-CDK. The antagonistic (i.e., opposing) relationship of Wee1 and Cdc25 was discovered using genetic experiments in which the “dosage” of the genes was experimentally manipulated. This simply means that the amount of protein present in the cell was increased or decreased depending on the mutation. Increasing the gene dosage (i.e., a gain-of-function mutation) increases the concentration of enzyme in the cell, whereas decreasing gene dosage (i.e., a loss-of-function mutation) leads to a decrease in enzyme concentration. The effects of these mutations on cell division are summarized in Table 08-02.
| The Effects of Mutations in WEE1 and CDC25 on Yeast Cell Growth and Division | ||
|---|---|---|
| Gene | Gain-of-Function Mutation* | Loss-of-Function Mutation** |
| WEE1—inhibitory kinase | Cells divide later at a larger-than-normal size | Cells divide early at a smaller-than-normal size (i.e., they’re “wee”!) |
| CDC25—activating phosphatase | Cells divide early at a smaller-than-normal size | Cells divide later at a larger-than-normal size |
| *i.e., too much protein produced or produced all of the time without regulation **i.e., no protein produced |
||
Specific Cyclins and Their Role in the Cell Cycle
As mentioned earlier in this topic, we are focusing on three specific checkpoints in the cell cycle. Of those, two are controlled directly by cyclin-CDKs: the G1/S checkpoint and the G2/M checkpoint. Each of these checkpoints is controlled by its own cyclin-CDK complexes. Cyclins and CDKs involved in the start of mitosis are called M cyclins. The progression into S phase is more complicated, and multiple cyclin/CDK complexes are needed (see Figure 08-03). Interestingly, the metaphase checkpoint is indirectly controlled by cyclin/CDK complexes. The M cyclin-CDK complex also activates the process by which the metaphase checkpoint is set up and then passed. We’ll look at how the different checkpoints work here.
Control of the G1/S Checkpoint and S Phase Progression
The transition from G1 to S phase is controlled by multiple cyclin-CDK complexes, including a G1 cyclin-, an S-cyclin-, and a G1/S cyclin-CDK complex (Figure 08-03). The G1/S cyclin-CDK complex will be de-activated once the checkpoint is passed, but the others will continue to function. Once the G1/S checkpoint is passed, DNA replication will begin.
One of the most important criteria for the passing of the G1/S checkpoint is that there can be no detectable DNA damage. The protein that manages checking for DNA damage is a transcription regulator called p53. p53 is sometimes called “the guardian of the genome” because of the vital role it plays in ensuring that DNA remains intact and undamaged. It also functions in a way that is somewhat counterintuitive—in its inactive state, p53 is constantly translated and then immediately degraded (Figure 08-06). When DNA damage is detected, p53 gets phosphorylated, which stops it from being degraded. It then binds to the promoter sequence for a CDK inhibitor called p21, thereby activating its transcription and subsequent translation. p21 blocks the activity of the G1/S cyclin-CDK complex, which will stop the cell from passing the G1/S checkpoint.
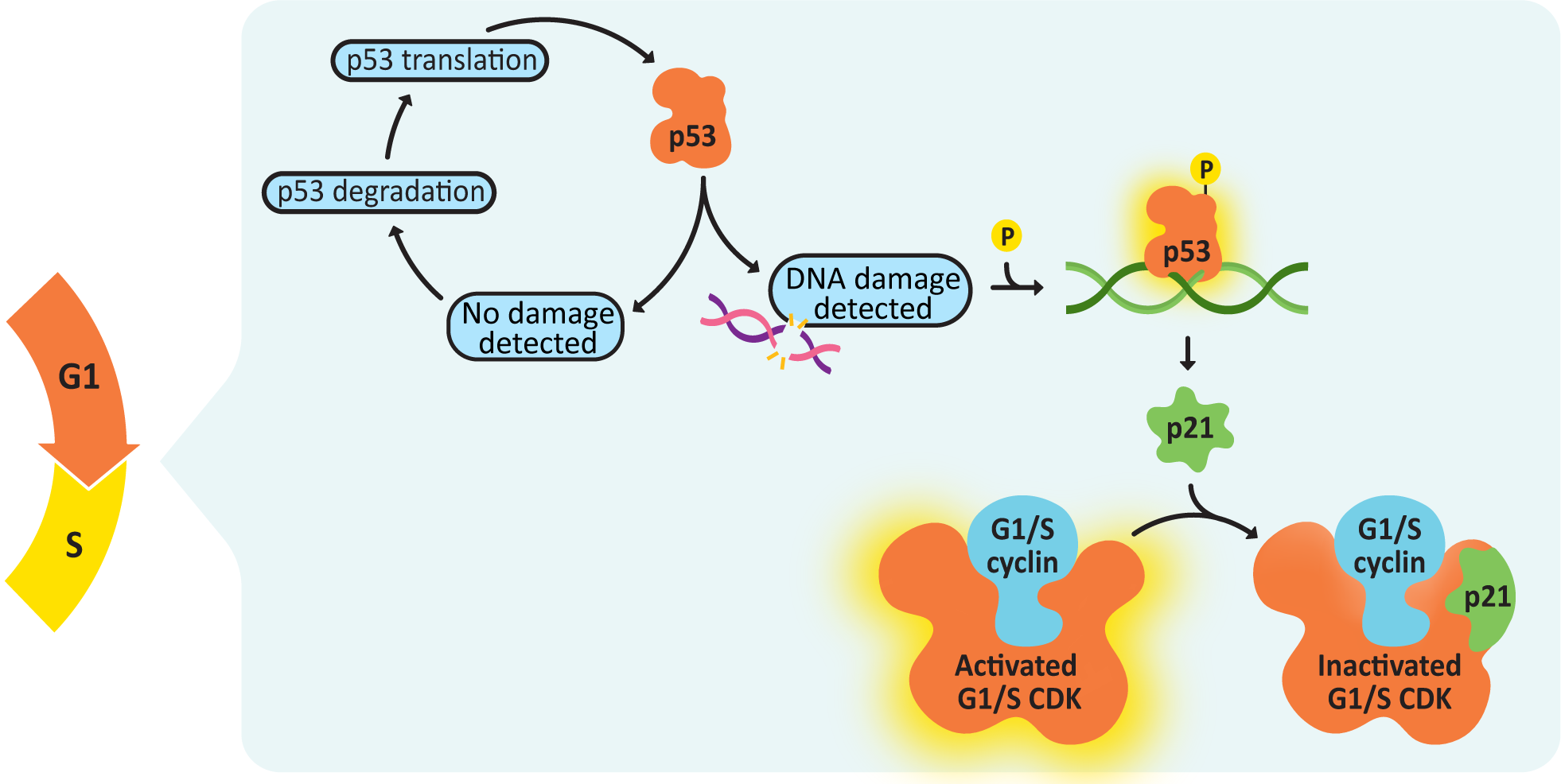
It is also interesting to note that p53 is mutated in as many as 50% of all cancers, which is a clear indication of just how important this protein is in the proper function of the cell cycle. If you lose p53 function in a cell, you lose your ability to delay the cell cycle so that there’s time to repair DNA damage. At that point, damage is less likely to get fixed before replication, and mutations accumulate at a much faster rate, with each new round of replication.
Once the G1/S checkpoint has been passed successfully, the active cyclin-CDK complexes help initiate S phase via phosphorylation of the replication machinery, which, in turn, helps it assemble on the DNA. DNA helicase—responsible for DNA unwinding—is also activated via phosphorylation, allowing replication to begin.
While there is much focus on how S phase is initiated, it is equally important to consider how it is terminated. DNA replication machinery must be deactivated at the end of replication as well. This ensures that the entire genome is replicated once, and only once, during S phase. Again, the cyclin-CDKs control this by phosphorylating key enzymes, which will lead to the eventual shutdown of replication.
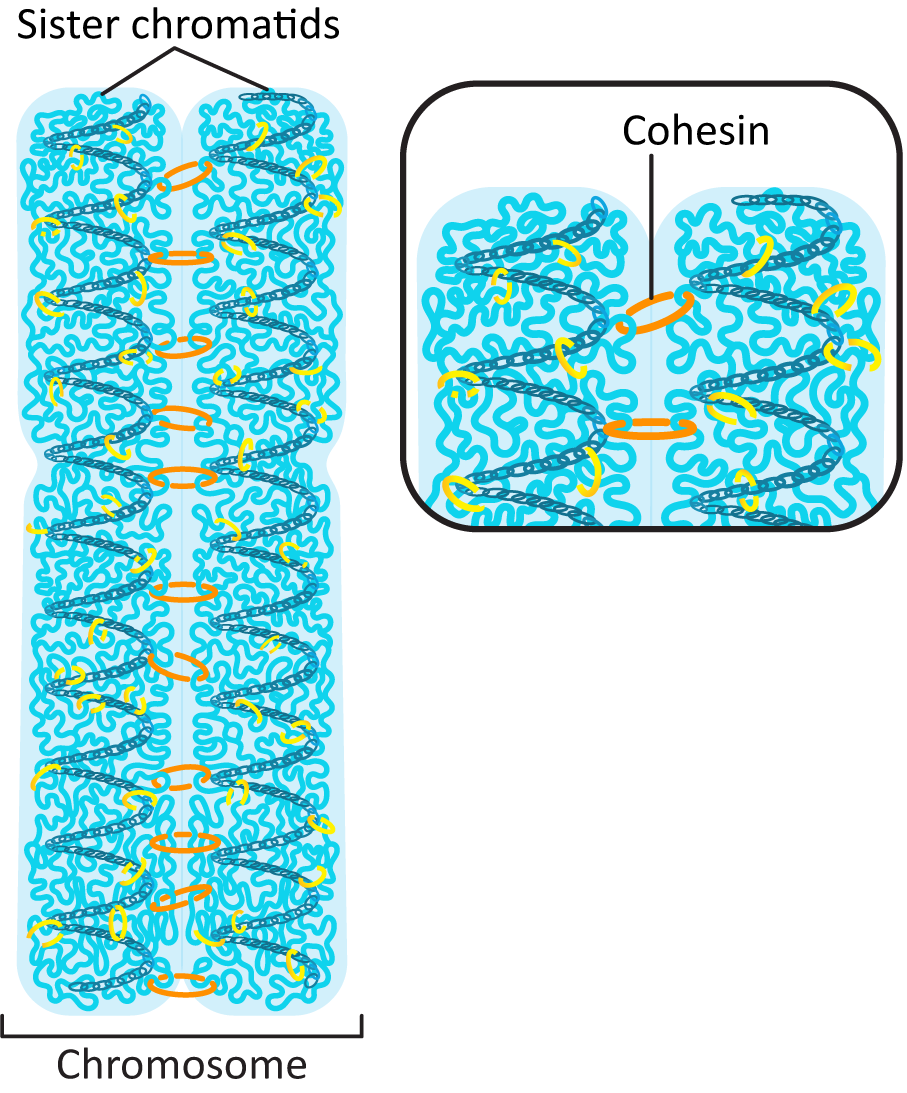
Prior to the start of S phase, each chromosome in the genome was made of a single helix of DNA that is in a complex with histones to form a chromatin fiber. (Review Chapter 3 if needed.) It has its own telomeres at each end of the chromosome and a centromere near the middle of the DNA. During DNA replication, the DNA double helix is duplicated, and the chromosome now includes two sister chromatids. This requires that a whole new set of histones is synthesized and imported into the nucleus to form the new chromatin fiber. Also, the two sister chromatids must be held together until the point at which they separate, during mitosis. This is done using a protein we’ve seen before (in Chapter 3) known as cohesin (Figure 08-07). Cohesin attaches the two chromatids together along their entire length at specific sites on the DNA called cohesin attachment regions (or CARs).
One interesting final point: S-CDK has been shown to remain active right until the start of mitosis (Figure 08-03) despite the fact that S phase is over long before that. The reasons for this are not entirely clear, but there is some evidence that S-cyclin-CDK helps with the activation of M-CDKs. This provides further continuity in the cell cycle and shows that the different cyclins are influenced by each other.
M-CDK Controls the G2/M Checkpoint and Reentry into G1
The transition from G2 phase to M phase is complex. It requires an almost complete rearrangement of the cytoplasm, including shutting down all transcription and translation, preparing all of the organelles for separation (which often means deconstructing them), building a second microtubule organizing center (MTOC) for the mitotic spindle, and completely rearranging the cytoskeleton to allow for division. M-CDK-cyclin becomes enzymatically active at the end of the G2 phase and is the primary control for this transition. M cyclin concentrations peak in metaphase, before crashing, which deactivates the M-CDK and marks the beginning of the transition back to G1.
As a kinase, the role of M-CDK is to phosphorylate other proteins, which, in turn, will activate or deactivate them depending on the protein. Some of the targets of the activated M cyclin-CDK complex include the following:
- Histone H1—Phosphorylating H1 leads to changes in chromatin configuration and, in conjunction with other proteins, leads to the tighter packing of chromatin required for mitosis.
- Condensins—These are a class of DNA-binding proteins that bind to chromatin to help with higher-order chromosome condensation. They work to loop up the chromatin fiber into the tightly coiled mitotic chromosome.
- Nuclear lamins—Phosphorylated lamins have a lower affinity for each other, and as such, the nuclear lamina falls apart. Disassembly of nuclear lamina results in the breakup of the nuclear envelope. (We explored this in detail in Chapter 3.)
- Structural proteins of the nucleolus—Since DNA from multiple chromosomes is used to form the nucleolus, it must be taken apart prior to mitosis. Phosphorylation of the structural proteins results in dispersion of the nucleolar proteins and disintegration of the nucleolus.
- A variety of protein kinases that regulate the cytoskeleton—In order for mitosis to happen, the cytoskeleton needs to be completely taken apart and rebuilt. A number of proteins are involved in this, including microtubule-associated proteins (MAPs) and even some actin-binding proteins (ABPs), and must be activated directly or indirectly by the M-CDK.
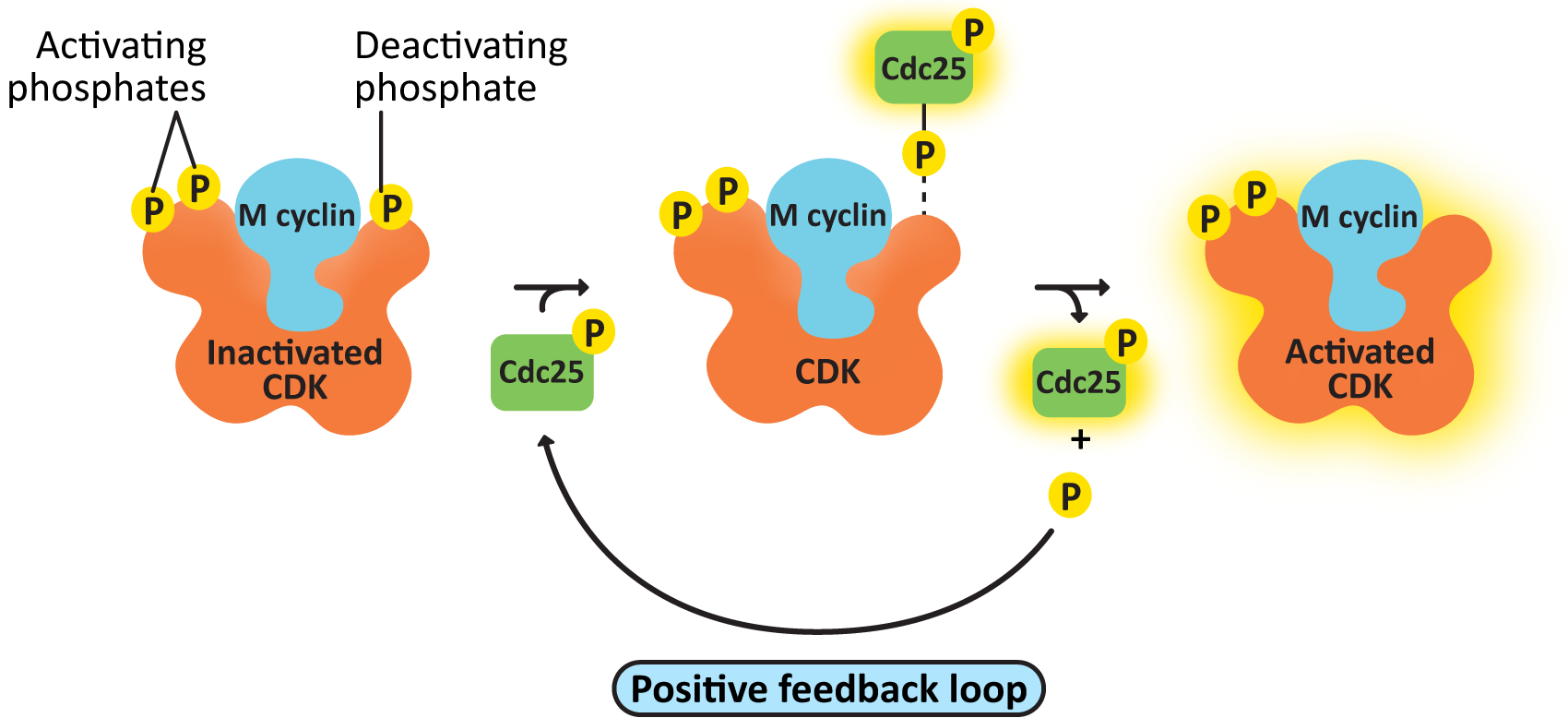
- cdc25, the M-CDK-activating phosphatase—This creates a positive feedback loop, resulting in further activation of M-CDK-cyclin. As a result, M-CDK activity rises increasingly rapidly as more M-CDK becomes active (Figure 08-08).
- Anaphase-promoting complex (APC)—This protein is key to passing the metaphase checkpoint. At the beginning of anaphase, APC degrades the cohesin proteins that bind sister chromatids together, releasing the daughter chromosomes. Interestingly, APC also activates enzymes that tag M cyclin for degradation. This ensures that M-CDK will be properly deactivated when its task is done. The cell cannot complete mitosis and return the cytoplasm to its interphase state unless M-CDK is inactive.
We will be breaking down the events of mitosis and how CDKs and other proteins drive that process in the next topic of this chapter.
The Metaphase Checkpoint Ensures Proper Mitotic Spindle Formation
Unlike the other two checkpoints, this checkpoint is not directly controlled by a cyclin-CDK complex. However, that doesn’t mean that the CDKs and cyclins have no role to play. This checkpoint is in the middle of M phase and ensures that the mitotic spindle is formed properly before mitosis is allowed to proceed. The M cyclins and their associated CDKs are involved in this checkpoint, as they activate the proteins required to create the mitotic spindle. Passing the metaphase checkpoint not only allows mitosis to proceed but also sets in motion a series of events that will eventually shut down M phase, which we will talk about in the next section.
Proper spindle assembly is essential to the metaphase checkpoint, so it stands to reason that the proteins involved in regulating this checkpoint would be interacting directly with the spindle itself. At some point during G2 phase, a large protein complex known as a kinetochore assembles at the centromere of each sister chromatid in the chromosome. This complex will help capture microtubules during the assembly of the mitotic spindle so that the sister chromatids can be separated. Several proteins are also assembled at the kinetochore, the most important of which is one called anaphase-promoting complex, or APC, and they will remain there until microtubules are properly attached to the kinetochores of both sister chromatids. Once that happens, the checkpoint proteins assembled at the kinetochore, including APC, are released and activated. Once that happens, the cohesins holding the sister chromatids together are degraded and anaphase begins.
This is not the end of APC’s role in mitosis, however.
Deactivation of the Cyclin-CDK Complex
Just like in S phase, the cell must eventually end M phase, put everything back where it was, and allow the new daughter cells to reenter G1. To do this, the M cyclin-CDK must be deactivated so that it stops phosphorylating its target proteins and the cell can exit M phase. APC is directly involved in the degradation of M cyclins, which deactivates the M-CDK.
Incidentally, that means that M cyclin-CDK not only creates a positive feedback loop to activate itself, through cdc25 (Figure 08-08), but it also creates a negative feedback loop, using APC, which will result in its eventual deactivation. This is a fascinating case study on the complexity of cell signaling and how it regulates cellular function.
Like most signaling events, the cyclin-CDK complex must be deactivated once its job is complete. This is done by a combination of
- shutting down the transcription and translation of new cyclin and
- degrading the cyclin proteins that already exist.
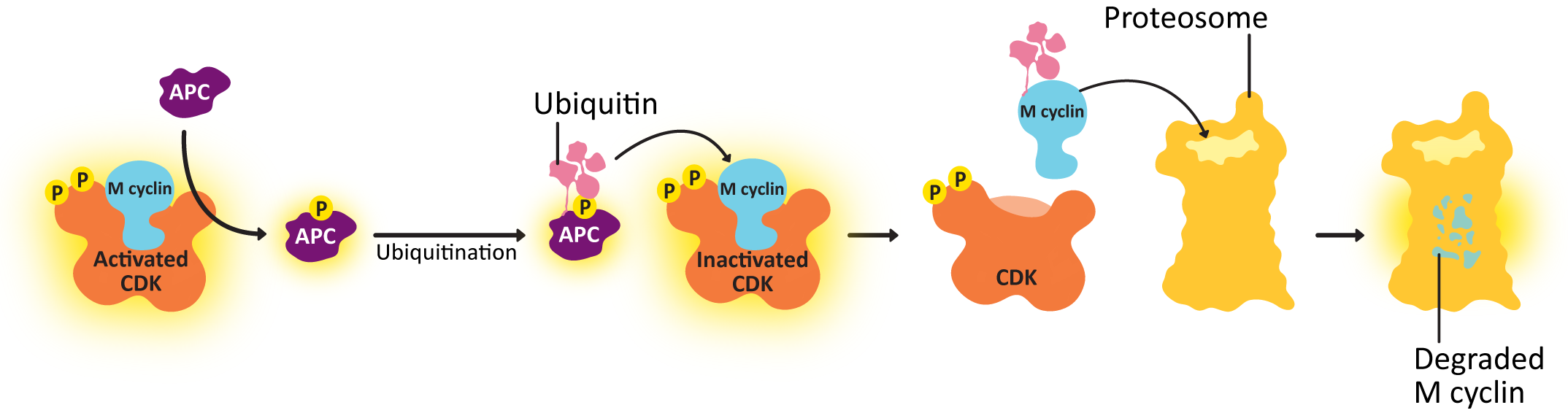
Negative feedback loops are often built into signaling pathways, and CDKs are no different. The mechanism is illustrated in Figure 08-09. APC is phosphorylated by M-CDK so that it is active and controls the metaphase checkpoint. Unlike many of the regulatory proteins we’ve seen, APC is not a kinase or a phosphatase. APC is a protein that transfers a small protein tag called ubiquitin to other proteins. (Review Chapter 4 if needed.) APC tags the cyclin with ubiquitin, which results in it being sent to the proteasome for degradation. Once the cyclin is degraded, the CDK is no longer active, and all of the phosphorylation targets of the CDK can then be dephosphorylated and returned to their original state. The result is that the concentration of cyclin in the cell drops rapidly, and the CDK is also deactivated, and the cell can start the process of putting the cytosolic contents back together again in the new daughter cells, complete cytokinesis, and return to G1. For its part, APC is deactivated in G1. It is one of the phosphorylation targets of the activated G1/S-cyclin-CDK complex.
Studying Cells: Experimental Techniques to Identify the Cell Cycle
Initially, we knew two things about the cell cycle: there was mitosis, and there was the time in between mitosis (i.e., interphase). We could observe this in the most rudimentary light microscopes roughly 200 years before we knew that DNA was the molecule that stored genetic information. As we now know, there are four major stages to the life cycle of a cell, and only one of them is mitosis. When researchers study the cell cycle, it is important that they can differentiate among the four stages. DNA content is a useful cue, since the amount of DNA changes in predictable ways throughout the cycle. In addition, sometimes researchers will need to work with a population of cells that are all in the same stage of development. In this section, we look at two ways to identify, and possibly synchronize, a population of cells based on cell cycle.
We will explore two different techniques and discuss each in turn:
- Chemically synchronizing cells in a population: This technique is the starting point for many experiments to ensure all cells are starting at the same stage in the cell cycle.
- Fluorescence-activated cell sorting (FACS): This is a subtype of a more commonly known procedure called flow cytometry, where cells are monitored for certain properties and grouped based on these properties.
Chemically Synchronizing Cells in a Population
When studying cells as they progress through the cell cycle, it’s common to want to take measurements based on a particular parameter. We can watch cells go through mitosis using a simple light microscope, but some of the other phases are more difficult to detect. As such, we may want to work with a population of cells that we know are all at the same phase. However, that generally does not happen naturally in a population of cells. Just like your classmates in elementary and high school hit their growth spurts at slightly different times even though you were all roughly the same age, cells will not naturally align their cell cycles.
In order to synchronize a population of cells, the scientist must apply some kind of block that stalls the cell cycle at a known step in the cell cycle. The two most common blocks are based on biological concepts we’ve seen before in this book.
- In Chapter 4, we discovered the existence of temperature-sensitive mutations, and how they could be used to study the essential proteins of the secretory pathway. Most cell cycle proteins are equally essential, as a cell that cannot progress through its cell cycle cannot divide. So temperature-sensitive mutants are useful in this context as well.
- In Chapter 6, we also saw that chemical inhibitors could be used to disrupt cytoskeletal function temporarily. This, too, is a strategy that can be applied to the cell cycle. There are a few known chemical inhibitors that block further progression in the cell cycle. DNA replication is often targeted by these compounds, and the cell stalls at the start of S phase as a result of the chemical inhibition.
When these blocks are applied, the cells will continue to progress through the cell cycle until they hit the blockage, and then they can go no farther. You can think of this like construction on a major bridge or roadway. The cars are able to move freely elsewhere as they work toward their destination, but once they arrive at the construction, they stop and stay there until the construction block is lifted so that the stopped traffic can again begin to move forward.
Fluorescence-Activated Cell Sorting (FACS)
This final technique is, in some ways, explained by its name. When we do FACS, we fluorescently label cells and then use that fluorescence to differentiate between cells that are in different states (Figure 08-10). The machine (called a flow cytometer) that measures the fluorescence is also able to separate a mixed population of cells based on this fluorescence (i.e., the cells are “sorted” into different groups based on the measured fluorescence).
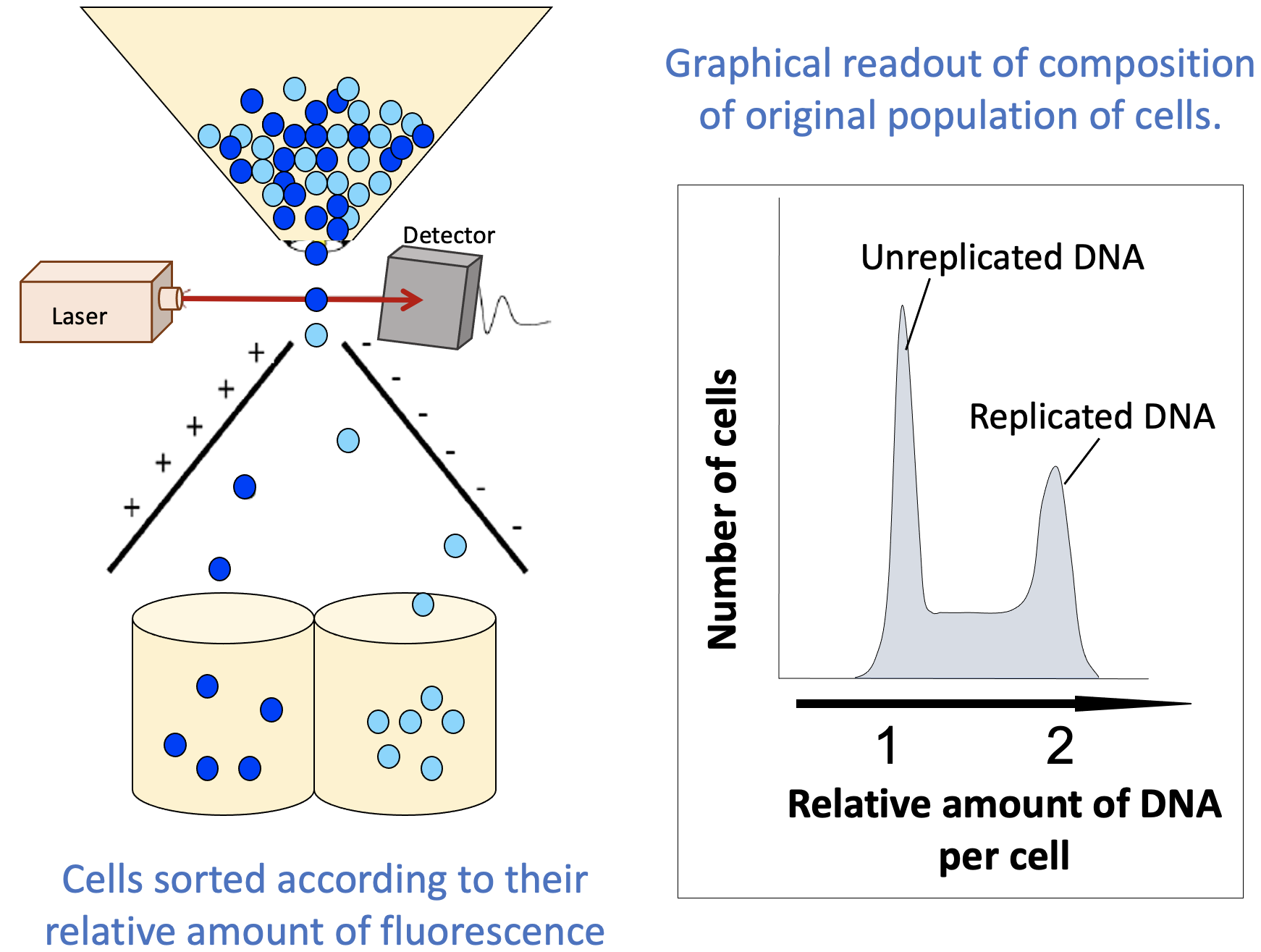
When using FACS to learn about the stage of the cell cycle that each cell in a population is in, a live fluorescent DNA stain like DAPI is commonly used. Since the amount of DNA changes as the cell cycle progresses, this is a very simple way to separate the cells in a sample. The result of this technique is twofold:
- You now have synchronized populations of cells without using chemical inhibitors or temperature-sensitive mutations. These can be used for further experiments.
- Cells in G1 will have one “full set” of their DNA, while cells in G2 and M phase will have duplicated their DNA (i.e., two “full sets”).
- Cells in S phase will have more than one set but less than two sets, as replication has started in S phase but is not yet complete.
- Additional visual separation may be required to differentiate between G2 and M phase, but this can be done relatively easily, as a cell undergoing mitosis can be identified using a standard light microscope.
- The flow cytometer also produces a graphical readout (Figure 08-10) that summarizes how many cells were found with each “amount” of fluorescent material (DNA in this case). These readouts can be used to learn important information about your population of cells.
Interpreting FACS Readouts
A simple FACS readout is shown in Figure 08-10. It is very much like a histogram, in which the y-axis represents the number of cells counted at each point on the x-axis (often listed as a percentage of the total population), and the x-axis measures the amount of DNA. (Note: This is usually done indirectly, by measuring the fluorescence intensity.) The amount of fluorescence intensity correlates to the amount of DNA in a given cell.
Since the amount of DNA in a cell correlates with the phase of the cell cycle, this readout can help us determine how many of the cells in our sample are in each stage of the cell cycle at the moment at which they were measured. We can compare cells at different time points, which might tell us whether our cells are able to progress through the cell cycle or whether they have stalled.
The following are some things to remember about FACS and the readout in Figure 08-10:
- The readout indicates “replicated” and “unreplicated” DNA, not whether it’s in G1, S, G2, or M phase. This is an important limitation of this technique. There are two phases in which the DNA has been replicated and as such are lumped together in the second peak (G2 and M phase). FACS cannot differentiate between these, since the DNA content is the same for both. Depending on the question you’re trying to answer, this might be key to correctly interpreting your results. To separate G2 from M phase in your sample, you would need to do something additional, like look at the cells in a microscope, for example.
- The part in between the peaks is more important than it might seem. In this area, we have more than one “full set” of DNA but less than two. There’s only one stage of the cell cycle where the amount of DNA goes from one set to two: S phase. Cells that are in the process of undergoing S phase will be found between the peaks that represent G1 cells and G2/M cells.
Like many of the techniques we’ve seen so far (FRAP, SDS-PAGE, etc.), FACS is a way to quantify what’s going on in your samples. It’s not actually the experiment itself. The experiment would be done before you put your samples through the FACS machine. If, for example, you wanted to explore the impact of a newly discovered mutation, you could compare the FACS readout from a wild-type (i.e., unmutated) and a mutated sample of cells and see how similar or different their FACS readouts are.
Changes in the FACS readout, even really strange and unexpected changes that don’t make a lot of sense at first glance, can tell us very important things about the samples and treatments we choose to study. Like all of the experiments we’ve covered in this book, understanding what the technique can, and cannot, reveal is essential to being able to accurately interpret the data you receive from your experiment.
Topic 8.2: Mitosis and Cell Division
Learning Goals
- Relate the progression of mitosis to the activation and deactivation of proteins by the mitotic CDK-cyclin complex.
- Explain the role of the cytoskeleton (and associated motor proteins) during mitosis and cytokinesis, including how dynamic instability contributes to the formation and function of the mitotic spindle.
- Describe how signaling events are used to end mitosis, starting at anaphase and followed by the transition of the cell back into G1.
The stages of mitosis are likely something you have been learning about since grade school, so it might seem odd at first that we are going to revisit them here. However, the reality of what actually has to happen, at the cellular level, in order for mitosis to occur is incredibly complex. As you may have guessed by now, nothing in the cell just happens by chance. It’s all driven by proteins acting on other molecules in the cell. For a cell to go through mitosis, here’s just a short list of cellular events that occur:
- The entire transcription and translation machinery needs to be shut down so that the DNA can be replicated, condensed, accurately divided, and then set up again in the new cell.
- The microtubule network in the cell needs to be dismantled and completely rearranged so that it can help with DNA separation.
- The actin network also needs to be rearranged to help with cytokinesis (division of the cytosol). This means that all of the regular work of the cytoskeleton during interphase (transport, organelle positioning, etc.) is also disrupted.
- Every organelle and structure in the cell must be duplicated and/or distributed into the two daughter cells so that both of the new cells have everything they need to survive. This often requires a complete dismantling of larger organelles (like the endomembrane system or the nucleus).
- A whole series of signaling events needs to be started up to initiate all of this change, and then, at the end, it all needs to be shut down again so that everything can transition back to the interphase and regular cellular function can be rebooted.
This incomplete list of events highlights the complexity of the process and also how vulnerable the cell is while this takes place. As long as the cell is focused on division, it cannot respond to changes in the environment or defend itself, metabolize new foods, or deal with damage. The cell needs to be efficient and get this work done quickly so that it reduces the time that it is vulnerable. The work also needs to be done very accurately, as any mistake in the process of mitosis is very likely going to kill one or both daughter cells.
Mitosis is a highly regulated dance that includes every part of the cell. This final topic of the book works to highlight some of the key elements of that dance and expand your understanding of the beautiful complexity involved in M phase.
The Hypercondensation of Chromosomes for Mitosis
Waaaay back in Chapter 3, we discussed the structure of the genome in detail, including how the DNA that forms each of the chromosomes is organized and compacted with the help of histone proteins. In this chapter, we will look at how the cell transforms the organization of the DNA in the interphase nucleus into the hypercompacted mitotic chromosomes that are required for cell division.
Just like we did in Chapter 3, we want to remind you that this is an area of active research that is extremely difficult to study, so we don’t have all of the answers for how this process works. We will do our best to tell you what science currently believes to be true and also what we still have yet to discover.
There are two parts to chromosome management during mitosis: The first is the actual condensation of the chromosomes at the start of mitosis. The second is the maintenance of the structure of the mitotic chromosome throughout the stages of mitosis, including the separation of the sister chromatids at anaphase. Remember that chromosomes are, in essence, extremely long strands of DNA that could easily become a tangled mess in the dynamic environment of the cell during mitosis. Based on what we currently know, it appears to use at least some (but not all) of the same proteins that are used to maintain interphase packing of DNA. We’ll focus primarily on the formation of the mitotic chromosomes and highlight what we know about the maintenance of these structures as we discuss the proteins involved.
Condensation of the chromosome for mitosis is thought to require only five additional proteins beyond the histones used to form the chromatin fiber. They are condensin I and II, cohesins, a kinesin known as Kif4A, and an enzyme called DNA topoisomerase II alpha. The roles of Kif4A and DNA topoisomerase II alpha are not entirely clear, even though it is clear that without them the mitotic chromosome is unable to form. From the histones, a key player in chromosome condensation for mitosis appears to be histone H1, also known as the linker histone. We’ll start our discussion with the H1 histone, as it connects the most directly with what we learned about interphase chromatin in Chapter 3.
Phosphorylation of Histone H1 Promotes Higher-Order Chromatin Packing
While the structure of histone H1 is slightly different from the core histones, it still has some key features that are common to all histones. For example, it has two tail regions, which are sites where posttranslational chemical modifications (phosphorylation, acetylation, methylation) commonly occur. Histone H1 is a key phosphorylation target for M-CDKs. Phosphorylation is thought to have a few different effects on the structure of the chromatin, which helps it begin the process of disassembling the interphase organization so that the mitotic chromosome structure can take shape. Our discussion of H1 in Chapter 3 showed us that H1 is often used to help pack up the chromatin more tightly, which reduces access for gene expression, so the involvement of histone H1 in preparing the DNA for mitosis makes quite a bit of sense.
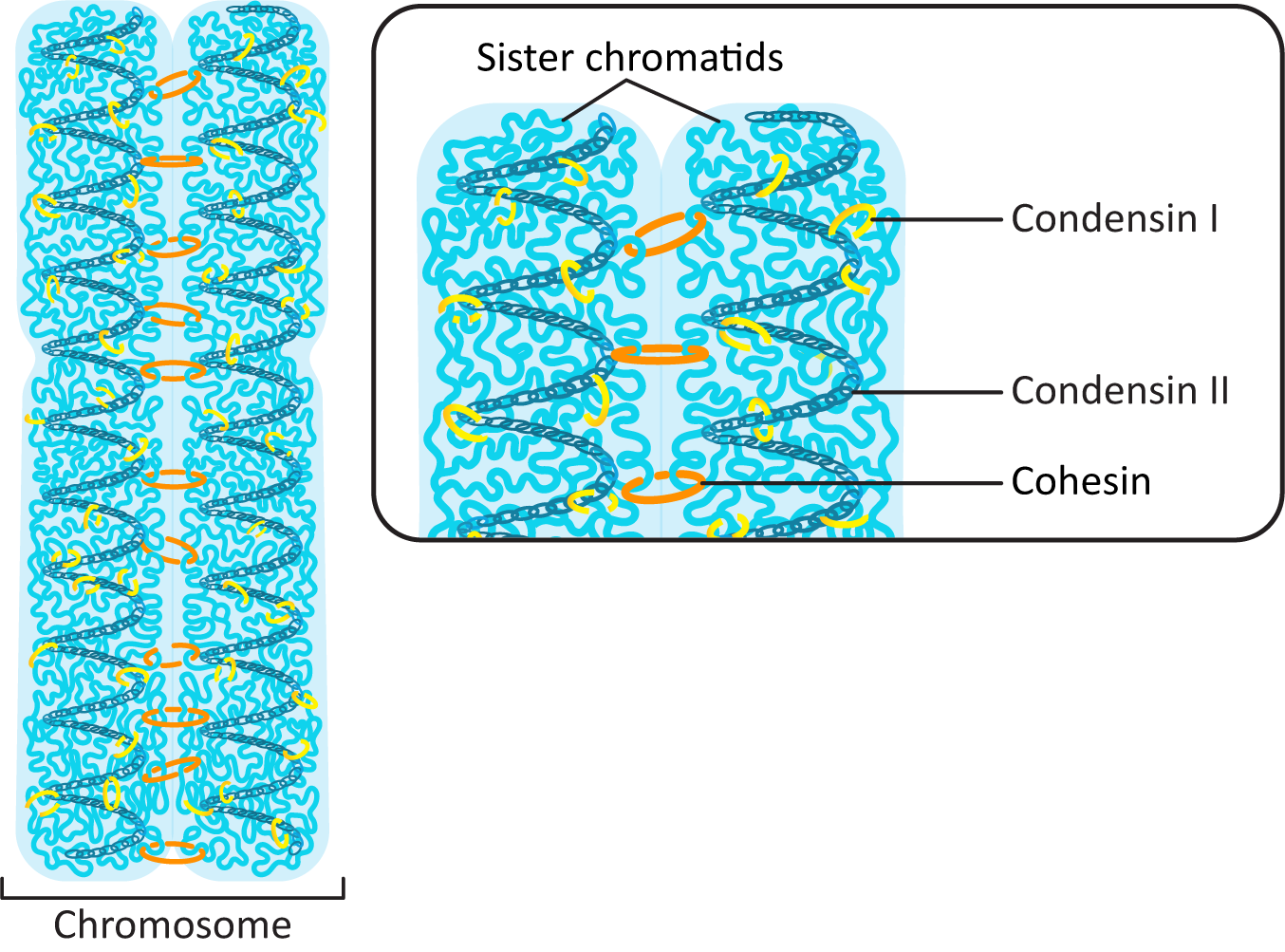
Condensin I and Condensin II Work Together to Pack the DNA into a Tight Column
The major work of packing the DNA up into the tight column that forms the mitotic chromosomes falls to two proteins that are part of the condensin family. Condensin I and II each have their own role to play in the process (Figure 08-11 and Video 08-03).
Cohesins Hold the Sister Chromatids Together
Interestingly, while cohesins have an important role in organizing interphase chromatin (they help form topologically associated domains, or TADs), they are removed from much of the DNA as the genome prepares for mitosis. Condensins pick up the slack and help loop up the DNA during the extreme condensation required for mitosis. However, cohesins still have an important function at this stage. As we saw earlier, cohesins are used to hold the sister chromatids together after replication, right until they are degraded during anaphase so that chromatid separation can take place (Figure 08-11).
The end result of all of this packing is an extremely condensed chromosome that is as much as 20,000 times more compacted than the original DNA strand. When observed in electron microscopy (Figure 08-12), the looped fibers of DNA can be observed as well as the sister chromatids and the centromere. This level of packing is so extreme that no transcription is possible.
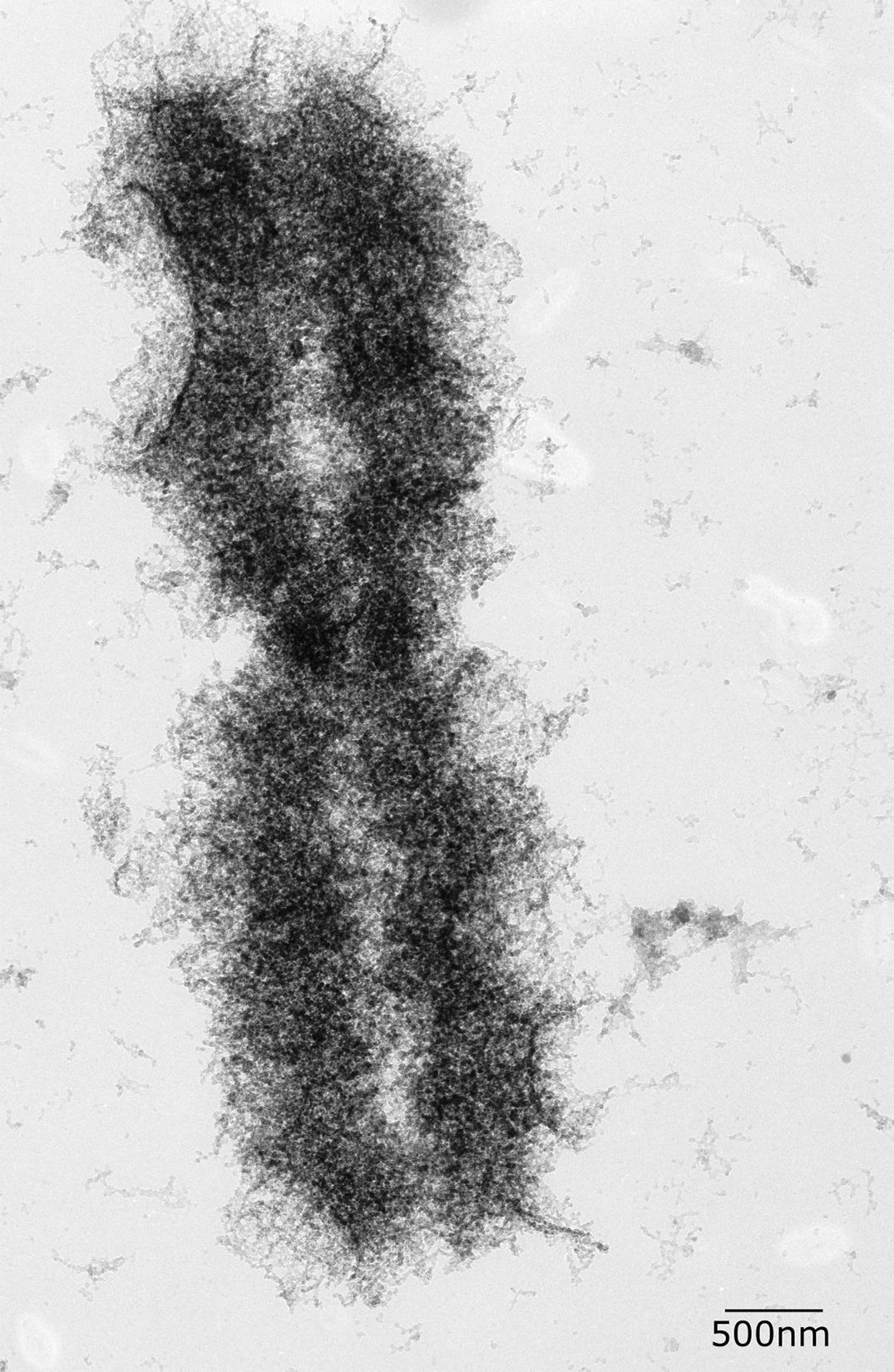
M Phase: Mitosis and Cytokinesis
M phase consists of two separate events:
- Mitosis: This is the process of division of the nucleus and its contents (i.e., the DNA!).
- Cytokinesis: This is where the rest of the cytoplasm gets divided into two new cells.
Both events are managed and controlled by a variety of proteins, which are activated and deactivated by the CDKs. It’s also important to note that the cytoskeleton plays a key role in both events.
To start, we’re going to take a step back and look at the process of mitosis through a microscope (Video 08-04).
It is likely that you have already learned the names of the stages of mitosis, as they are taught in elementary and/or high school. However, they are summarized in Figure 08-13.
We are going to look at each of these stages in turn. Our primary focus will be the rearrangements of the cytoskeleton that control the cellular events and the key signaling events that are responsible for triggering different parts of the process.
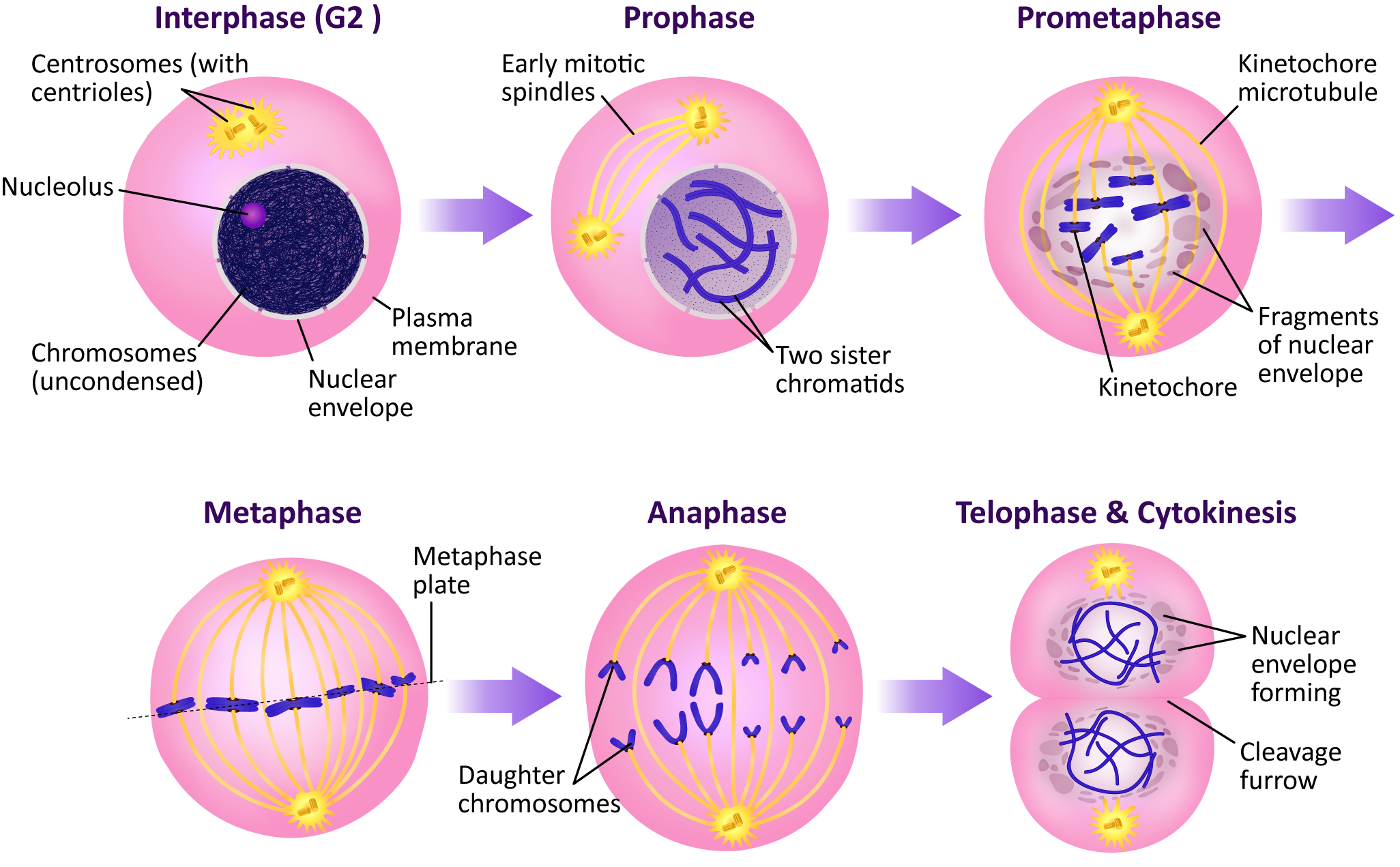
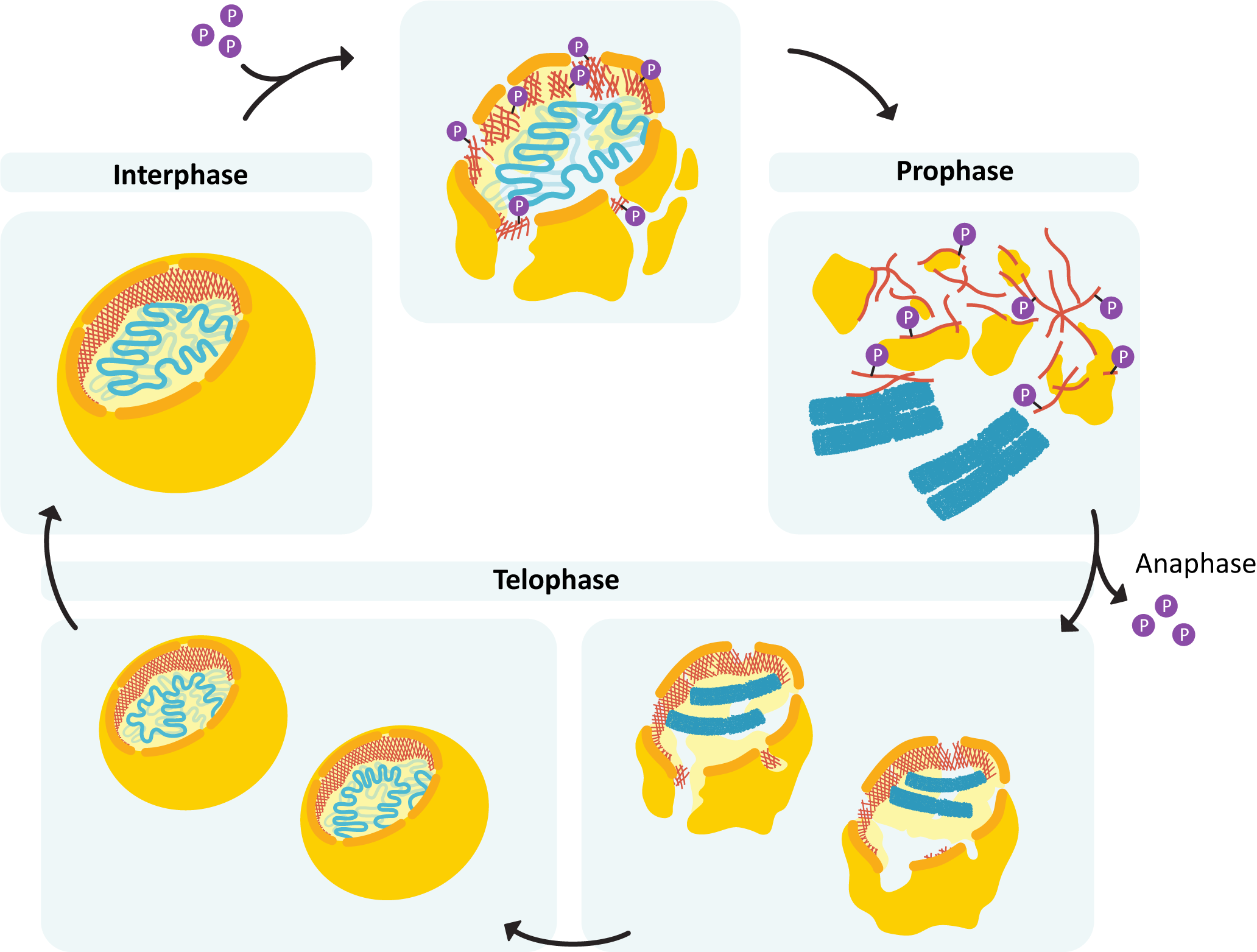
Mitosis Stage 1: Prophase (and Prometaphase)
Prophase is the first stage of mitosis. At this point, the cell has passed the G2/M checkpoint, and its M-CDK is fully activated, so it begins to prepare itself in earnest for mitosis. Each of these events is the result of M-CDK phosphorylating one or many of the targets mentioned earlier in this chapter.
- Chromosomes condense as H1 and nucleolin are phosphorylated.
- The nuclear lamins depolymerize, resulting in the nuclear envelope breaking apart.
- The mitotic spindle will begin to form.
The first step in mitotic spindle formation is the production of two centralized microtubule organizing centers (MTOCs) in the cell, which will become the opposite poles of the spindle. The process of spindle formation actually begins well before the start of M phase. As early as the transition to G2 phase, some of the components of the mammalian MTOC (also called the centrosome) will duplicate. At the onset of prophase, the centrosome splits in two, and the two MTOC will begin to migrate to either side of the nucleus. Interestingly, not very much is currently known about how plant cells transition from a decentralized gamma-tubulin distribution to the highly organized mitotic spindle.
There is more than one model for how the mitotic spindle is formed. Here we are going to focus on one called the “search and capture” model (Figure 08-14). In this model, the formation of the mitotic spindle relies very heavily on the dynamic instability of the microtubules. As microtubules grow outward from the spindle pole, they “search” the cytosol for things to grab onto (chromosomes, the plasma membrane, or microtubules from the other pole of the spindle). As you will remember from Chapter 6, microtubules are highly unstable unless capped or stabilized in some way. The formation of the mitotic spindle uses this characteristic to its advantage—a microtubule that doesn’t “capture” anything in the cytosol will depolymerize. As such, microtubules will grow and shrink at random until they encounter the proper components to form the mitotic spindle.
As the components of the spindle are stabilized and come together, three types of microtubule structures begin to form (Figure 08-15):
- If microtubules from opposite poles happen to interact with each other, they will stabilize each other (with the help of motor proteins), thus forming a bridge between the two spindle poles. These are aptly named interpolar microtubules, as they span the two poles.
- Conversely, if they happen to interact with a chromosome’s kinetochore during their random growth and shrinkage, they will attach and become a kinetochore microtubule.
- Astral microtubules, the third type of microtubule, are anchored at the plasma membrane (also with the help of motor proteins).
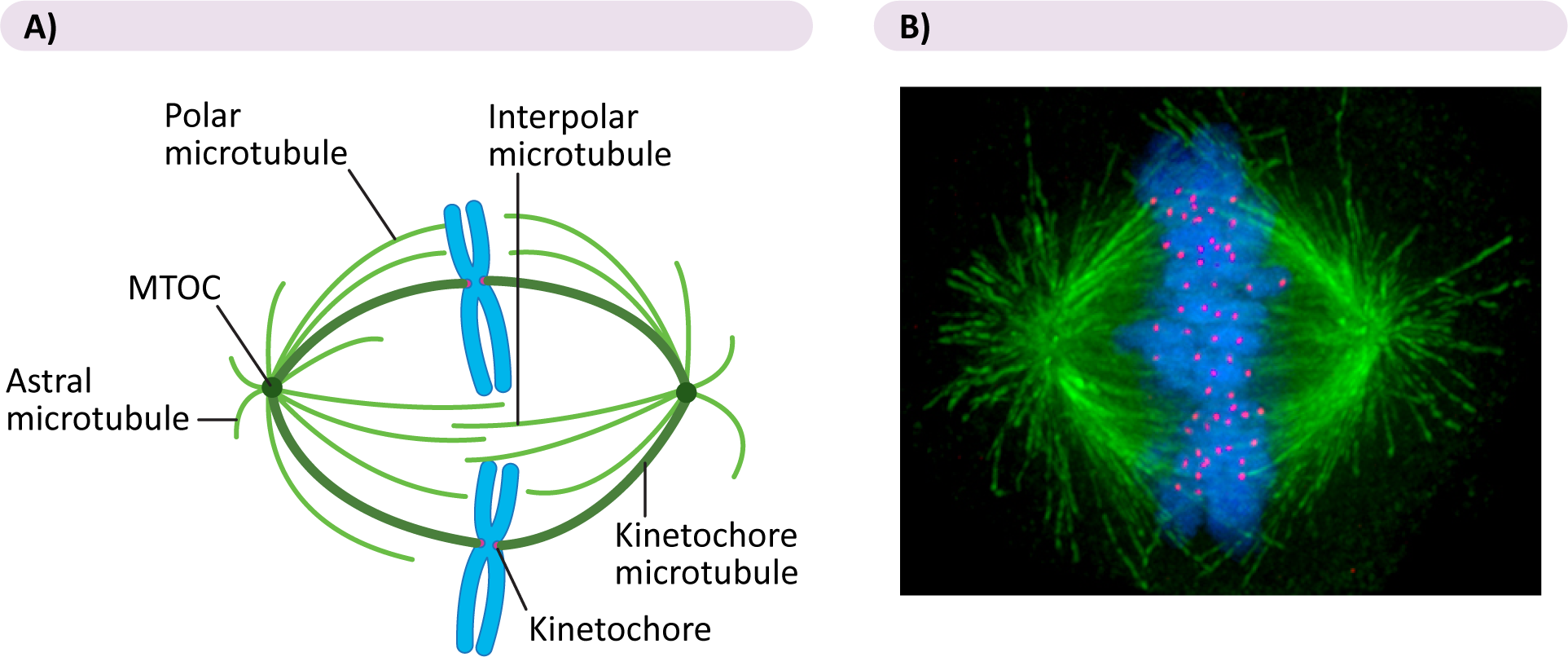
Though there is much that is still unknown about the formation of the mitotic spindle, scientists do know that motor proteins are absolutely crucial to this process. Several motor proteins have been identified as having a role in the formation of the mitotic spindle, including the following:
- Kinesins are found at the kinetochore and help the chromosomes stay attached to the kinetochore microtubules as they disassemble in anaphase.
- Dyneins hold the astral microtubules and also help pull the spindle poles apart in anaphase
- Interpolar microtubules have a special double-headed kinesin, which walks along each of the interpolar microtubules in a set. They also help to push the spindle poles apart in anaphase B.
There is a distinction sometimes made between the beginning and end of prophase. We often say that once the nuclear envelope breaks down, we have entered prometaphase. The breakdown of the nuclear envelope is an important event, as the mitotic spindle cannot be completed until that happens and the condensed chromosomes are free in the cytosol. It is only then that the “search and capture” part of spindle formation can begin in earnest.
By the end of prometaphase, each of the chromosomes is held at the center of the mitotic spindle by microtubules attached to the kinetochore. The kinetochore is a specialized region of the chromosome associated with the centromere. As you may recall, the centromere is a DNA sequence that is the site of assembly of the kinetochore structure. The kinetochore is a plaque or button-shaped structure composed of proteins. There is one kinetochore associated with each of the chromatids, and the microtubules get inserted into them. They face in opposite directions on the two sides of the chromosome.
Mitosis Stage 2: Metaphase
After the nuclear envelope breaks down and the chromosomes are attached to the mitotic spindle by the microtubules, we transition to metaphase. Notably in this phase, the chromosomes align on the metaphase plate—an imaginary plane equidistant from the two spindle poles. To accomplish the chromosomal alignment, chromosomes are pulled simultaneously toward both spindle poles by the kinetochore microtubules. Even tension on the chromosome from both sides of the spindle is required for everything to stabilize and stay in place. The proper tension is only achieved when the chromosome is properly aligned at the metaphase plate.
It is known that even though both ends of the microtubules are being held in the mitotic spindle, the state of kinetochore microtubules is still very dynamic—tubulin units are continually being added and removed at both ends. This is intriguing and unusual, considering that each microtubule is held at the minus end by the MTOC (presumably with the help of gamma-tubulin), and the plus end is held by the kinetochore. While it is not entirely clear how the minus end can both be held by the MTOC and allow for dynamic instability, the story of how this works at the kinetochore is taking shape. Video 08-05 summarizes how we think it works. Something worth noting is the release of the metaphase checkpoint signal, which is the signal to activate the anaphase-promoting complex (APC) and initiate anaphase. This event leads us to the next stage of mitosis.
Mitosis Stage 3: Anaphase
Sister chromatids do not remain associated with each other by accident—they are held together by cohesins. At anaphase, the cohesins are dissolved, which allows the sister chromatids to separate. The abrupt destruction of the cohesin linkage between sister chromatids is brought about by activation of APC by M-CDK. Remember that APC’s function is to tag proteins with ubiquitin, marking them for degradation via the proteasome (Figure 08-16). APC first acts on a regulatory protein called securin, which then releases an enzyme called separase. Separase cleaves the cohesins, allowing the sister chromatids to separate.
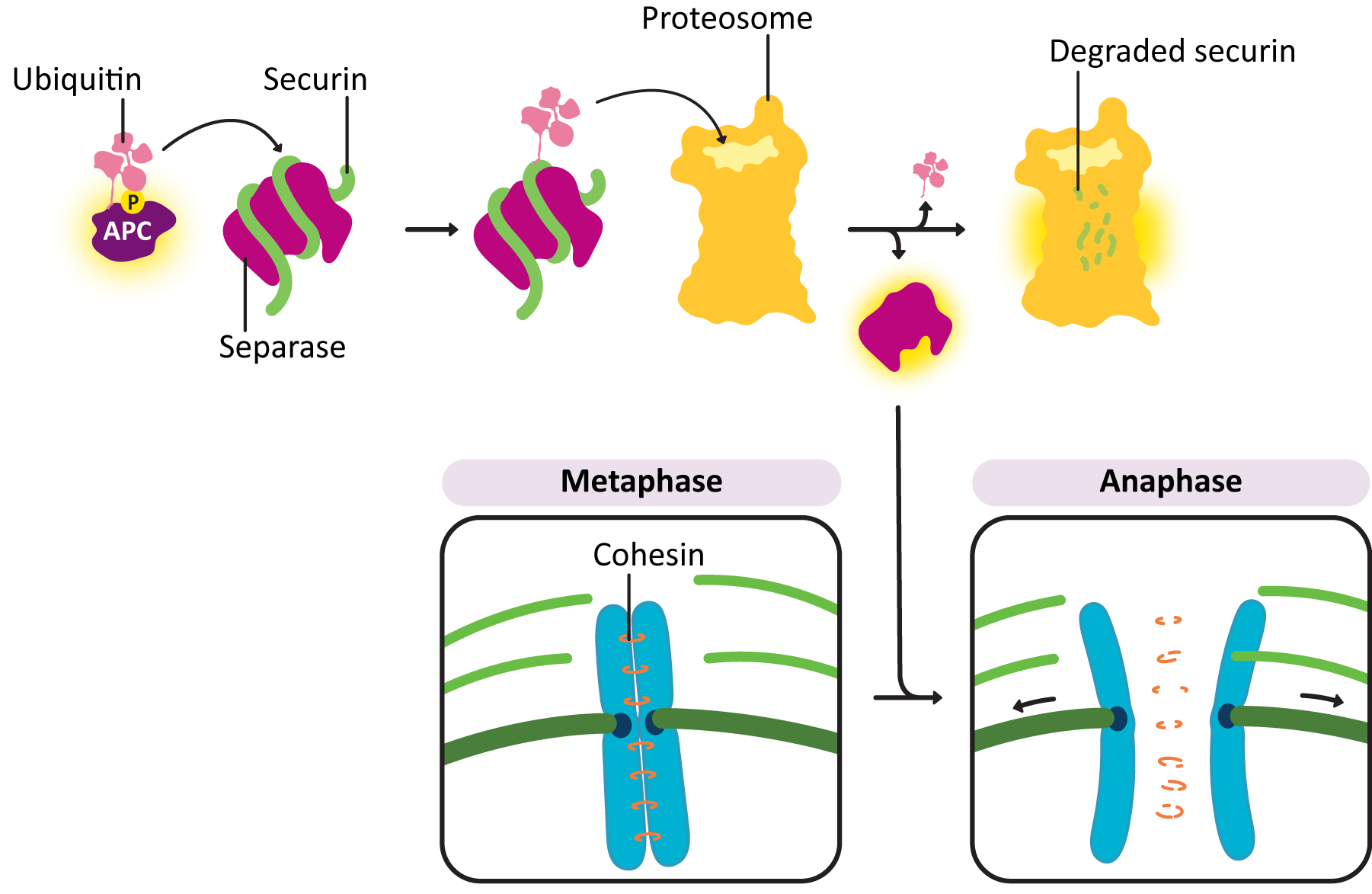
The movement of kinetochores toward the spindle poles in anaphase takes place through the action of motor proteins. The motor proteins that helped with spindle assembly now work to separate the sister chromatids from each other. In addition, the kinetochore microtubules are depolymerizing rapidly from their plus end. These two forces work together to separate the sister chromatids and move them to the opposite poles.
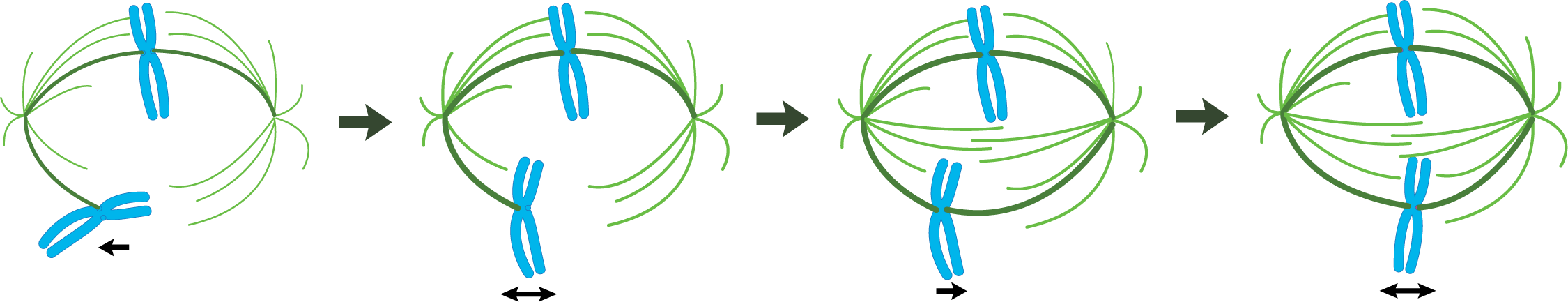
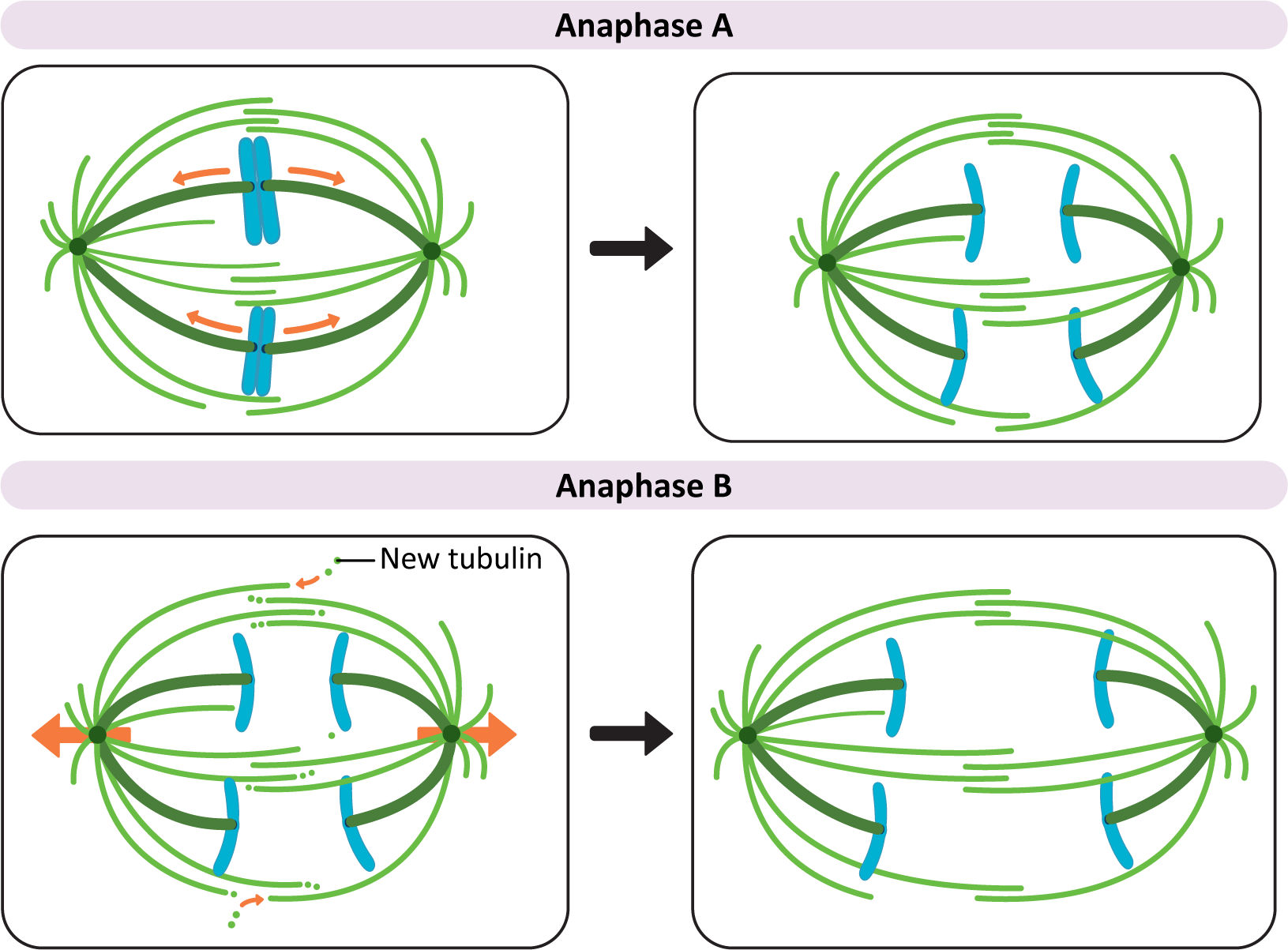
Anaphase movement of chromosomes is separable into two phases with different mechanisms: anaphase A and anaphase B (Figure 08-17):
- Anaphase A. During the initial part of anaphase, kinetochore microtubules shorten and move the chromosomes toward the opposite poles. This requires a combination of depolymerization of kinetochore microtubules and the action of motor proteins at the kinetochore that help keep the chromosome connected to the shrinking microtubule.
- Anaphase B. During anaphase B, the length of the kinetochore microtubules (MTs) remains more or less constant. Now it is the other MTs of the mitotic spindle that begin to do the work. Again, there is much that is still unknown about this process, but three separate forces have been shown to play a role in anaphase B:
- Tubulin subunits are added to the plus ends of the interpolar microtubules, making them longer.
- Motor proteins move the overlapping interphase microtubules from the two poles apart to make the spindle longer. This requires the help of the kinesins that are holding the paired interpolar microtubules together.
- Dyneins, attached to the astral microtubules and the cell cortex, walk toward the spindle poles on either side. This results in a shortening of the distance between the poles of the spindle and the plasma membrane. It also helps pull the spindle poles farther apart from each other.
Mitosis Stage 4: Telophase
By telophase, the division of the nuclear contents is now complete; thus the cell needs to shut down mitosis and prepare for entry into G1. Several processes occur:
- The nuclear lamina must reassemble so that the nuclear envelope can be rebuilt around the chromosome.
- The mitotic spindle disintegrates, and the cytoskeleton reassembles into its interphase conformation.
- The chromosomes must decondense and reform their interphase arrangement, which includes the reassembly of the nucleolus.
If you examine the above list, you should notice that this is the reverse of what happened in preparation for mitosis and prophase. Since preparation for mitosis requires the activation of M-CDK, it makes sense that all of these changes to prepare for G1 are a result of the deactivation of the same M-CDK. APC shuts down M-CDK by tagging M cyclin with ubiquitin. This results in the destruction of M cyclin by the proteasome (Figure 08-09). Once the cyclin is gone, the CDK can no longer function and is turned off. Because of this, the balance between active kinases and phosphatases is drastically shifted in favor of the phosphatases, which remove the phosphates that were previously added to M-CDK and its target molecules. This allows the proteins to return to their pre–M phase state, which allows the cell cycle to reenter G1.
As a direct result of the M-CDK shutdown, the nuclear lamina gets dephosphorylated and begins to reform around the chromosomes (Figure 08-18). The chromosomes retain their connection to the nuclear lamina throughout the cell cycle. As such, the chromosomes themselves provide a template for nuclear envelope reformation, ensuring that the nucleus reforms without excluding any of the chromosomes. APC continues to be active throughout the end of M phase and into G1, when it gets deactivated by the G1/S-cyclin-CDK complex.
M Phase Final Stage: Cytokinesis
Once the division of the nuclear contents is complete, the rest of the cytoplasm also needs to be divided between daughter cells. This occurs during cytokinesis, which is also the final stage of M phase (Figure 08-19). This process happens quite differently in plants, animals, and yeast and other fungi. However, in all cases, actin is the cytoskeletal fiber that plays the most important role.
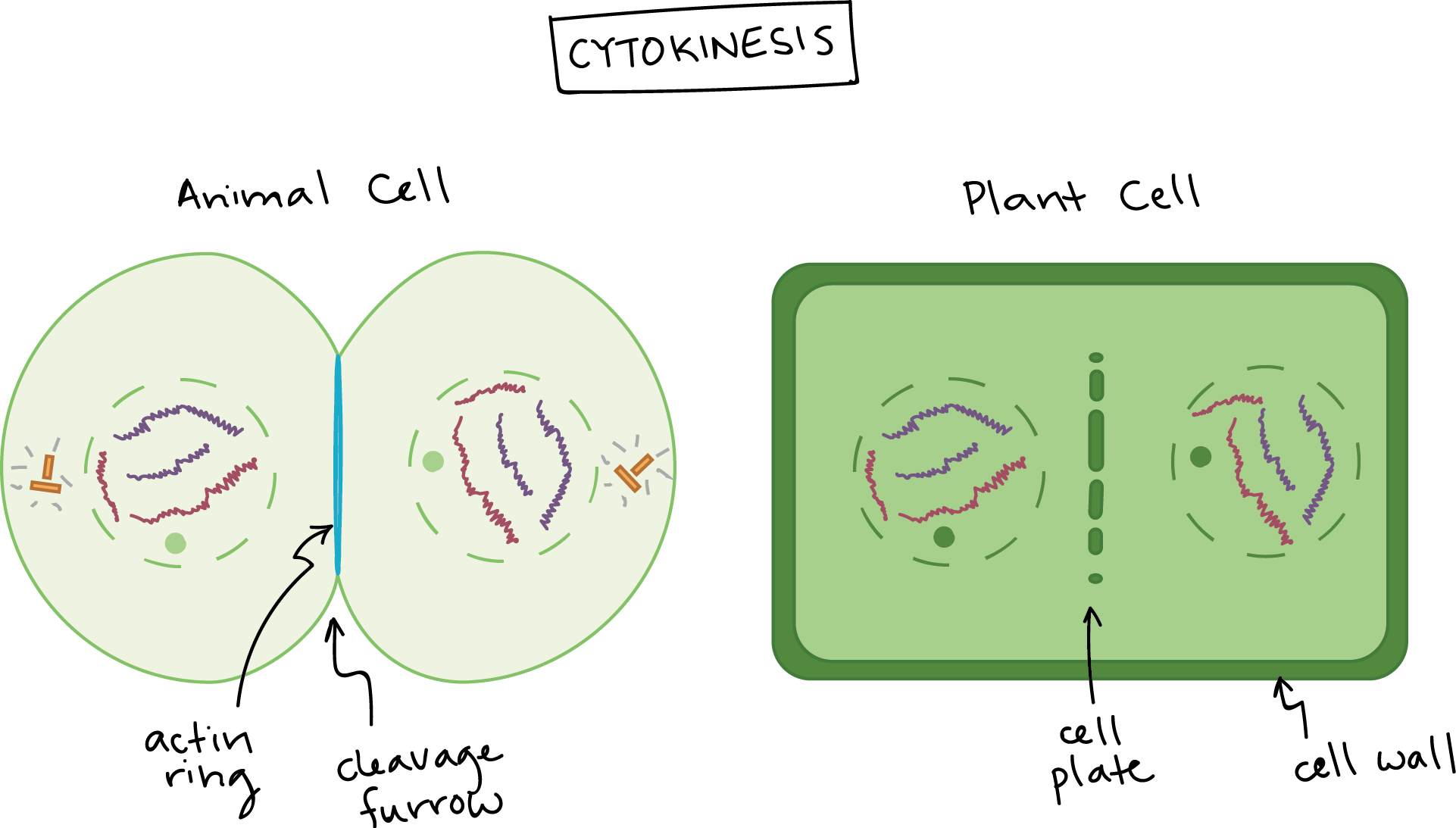
Cytokinesis in Mammalian Cells
In mammalian cells, actin arranges itself in an antiparallel array right underneath the plasma membrane. Together with myosin motors, they help form a contractile ring that pulls the plasma membrane inward and eventually splits the cell in two. Like many other parts of M phase, there is still much that is uncertain about the process of cytokinesis. However, the contractile ring has been found to always form around the midzone of the mitotic spindle, where the original metaphase plate was found. Thus, it is believed that the mitotic spindle itself plays a role in determining where the contractile ring will form.
As the myosin walks along the actin filaments of the ring, the ring contracts around what’s left of the mitotic spindle (namely, the interpolar MTs). The organelles have been either broken down into vesicles (ER and Golgi) or duplicated (mitochondria) to ensure that there will be more or less equal quantities of them in each of the daughter cells. Thus, dividing the cytoplasm also divides the organelles. At the same time, vesicles fuse rapidly with the plasma membrane, which helps ensure that there is enough plasma membrane to fit around each daughter cell.
Cytokinesis in Plant Cells
In plants, the cell not only must divide its cytoplasm, but a new cell wall must be produced between the two daughter cells. As a result, the Golgi apparatus, a key synthesizer of cell wall components, remains functional throughout. The many small mobile plant Golgi do not vesiculate (i.e., break into small pieces) like they do in animal cells but instead divide by fission prior to mitosis. Electron microscopy shows that they congregate on either side of the site of the new cell wall. The interpolar microtubules also help guide this process through the formation of a structure known as the phragmoplast in the center of the cell. The Golgi secrete cell wall compounds very rapidly to the center of the phragmoplast to build a cell plate. Actin has an important role to play in forming the phragmoplast and directing the vesicles to the cell plate, though the exact mechanism is still unclear. The cell plate grows from the center of the cell toward the outer walls and then fuses with the side walls of the dividing cell. At that point, the new daughter cells have been fully formed with their full complement of organelles and DNA.
Mitosis and Cytokinesis in Other Eukaryotes
The information in this chapter covers the most commonly discussed forms of cytokinesis—namely, plants and animals. It’s important to remember that much like every other process in the cell, Eukaryotes are a hugely diverse set of organisms, which means that mitosis and cytokinesis can vary quite a bit from what is described in this chapter. In addition, specific tissues within organisms will vary. While we don’t have time to go into all of the different ways that mitosis happens, we did want to end this chapter with a few interesting examples of how different it can be:
- Plant and animal cells undergo what’s called open mitosis, which means that the nuclear envelope breaks down completely in order to form the mitotic spindle. There are also two other forms:
- Partially closed mitosis occurs when the nuclear envelope remains intact and the spindle is formed both inside and outside of the nuclear envelope. Budding yeast (Saccharomyces cerevisiae) uses a partially closed format, which is especially interesting considering how often they are used as a model organism for all Eukaryotes.
- A third form is closed mitosis, which happens when the nuclear envelope not only remains intact, but the nuclear pores are plugged to close the nucleus off completely from the rest of the cell. This is more common in protists, fungi, and other single-celled organisms.
- Many cell types don’t divide evenly at mitosis. Lots of plant and animal cells undergo asymmetric division, which results in one daughter cell that is larger than the other. Additionally, lots of cells “grow” their daughter cell off of one side and move the new daughter nuclei into the bud once the bud is large enough. Saccharomyces cerevisiae is called “budding yeast” for this very reason. There are many additional cells that do this, especially single-celled algae, fungi, and other protists.
Chapter Summary
As you can tell now that you are at the end of this chapter and the end of the book, the cell cycle is truly a great process to integrate all the knowledge that you have gained thus far. In addition, we hope that you appreciate the additional layers of complexity required to regulate one of the most important cellular processes.
The cell cycle is an intricate dance that requires the cooperation of most of the cellular systems. We’ve emphasized a few in this chapter. But we challenge you to think of even more connections to the other units in the book.
- Signaling: The cell cycle requires integrated information from the extracellular and intracellular environments through signaling. The balance of activating forces versus inactivating forces helps ensure that cells divide only when the time is right.
- Cytoskeleton: The cytoskeleton is highly involved, especially in M phase. It’s clear that dynamic instability, a hallmark of the cytoskeletal system, is vital for the formation of the mitotic spindle but also the correct alignment of the chromosomes at the metaphase plate.
- DNA packing: The molecular packers and organization help unpack all the DNA during synthesis and do the opposite in M phase, completely packaging the DNA.
- Other organelles: Of course, these organelles need to be properly divided between daughter cells, but that does not end their contribution to this process. Prior to shutdown for mitosis, they need to make sure that everything the cell will need is available. This will include a great deal of energy, in the form of ATP and GTP, membrane, vesicles, and even polysaccharides if a wall needs to be made between the two daughter cells.
With that, we’ve come to the end of the book. We congratulate you for getting to the end! No matter your starting place, we hope that you have at least a little more appreciation for the complex beauty of the cell.
Review Questions
Note on usage of these questions: Some of these questions are designed to help you tease out important information within the text. Others are there to help you go beyond the text and begin to practice important skills that are required to be a successful cell biologist. We recommend using them as part of your study routine. We have found them to be especially useful as talking points to work through in group study sessions.
Topic 8.1: Regulating the Cell Cycle: Checkpoint Control
- Make a list of the four phases of the cell cycle, and describe in detail what has to happen in each phase before it is complete. Be as specific as you can.
- Describe how to use FACS is used in experiments to understand the regulation of the cycle.
- Explain the concept of checkpoint control of the cell cycle and give examples of what types of conditions should be met before S and M phases proceed. Explain why those conditions need to be met before the cell moves to the next phase.
- Describe the sequence of events leading to CDK activation and deactivation.
- Define negative and positive feedback. How are they thought to play a role in cell cycle control?
- Discuss the role of CDK-cyclin complexes in regulation of the cell cycle. How do they contribute to the cell “knowing” the conditions are right for the next stage of the cell cycle?
- Compare and contrast the roles of CDKs that control entry into S phase versus entry into M phase. Make a list of the different examples of proteins that they are thought to interact with and their effects.
- Explain the role of phosphorylation in regulation of the cell cycle. Explain how phosphorylation can affect protein folding and how that might result in changes in protein activity.
- Draw a FACS graph for the following groups of cells:
- Cells arrested in G1
- Cells arrested in G2
- Cells arrested in S phase
- Cells arrested in M phase
Topic 8.2: Mitosis and Cell Division
- Describe the role of dynamic instability in the formation and maintenance of the mitotic spindle.
- Illustrate with labeled diagrams the relationship between chromatids and chromosomes at each of the stages of the cell cycle.
- Explain how each stage of mitosis is ultimately controlled by the activation and deactivation of CDKs.
- Compare and contrast the following stages:
- prophase and prometaphase
- prophase and telophase
- anaphase A and B
- What is cytokinesis, and how does it differ in plants and animals?
A cellular process of nuclear division.
The process of cytoplasmic division.
“The series of events that take place in a cell that causes it to divide into two daughter cells.” Source: Cell cycle. (2023, June 7). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Cell_cycle
“The first of four phases of the cell cycle that takes place in eukaryotic cell division. In this part of interphase, the cell synthesizes mRNA and proteins in preparation for subsequent steps leading to mitosis. G1 phase ends when the cell moves into the S phase of interphase. Around 30 to 40 percent of cell cycle time is spent in the G1 phase.” Source: G1 phase. (2022, December 19). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/G1_phase
A discrete stage in the cell cycle where the whole genome is duplicated.
“The third subphase of interphase in the cell cycle directly preceding mitosis. It follows the successful completion of S phase, during which the cell’s DNA is replicated. G2 phase ends with the onset of prophase, the first phase of mitosis in which the cell’s chromatin condenses into chromosomes.” Source: G2 phase. (2022, December 19). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/G2_phase
The stage of the cell cycle where mitosis and cytokinesis occur.
All phases of the cell cycle that are not mitosis or meiosis. This stage contains G1, S, and G2 phases.
“During terminal differentiation, a precursor cell formerly capable of cell division permanently leaves the cell cycle, dismantles the cell cycle machinery and often expresses a range of genes characteristic of the cell’s final function (e.g. myosin and actin for a muscle cell).” Source: Cellular differentiation. (2023, April 25). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Cellular_differentiation
Molecular mechanisms that ensure that proper cellular conditions are met prior to allowing a cell to enter the next phase of the cycle.
A cell cycle checkpoint that regulates the progression of a cell into the S (synthesis) stage of the cell cycle.
A cell cycle checkpoint that regulates the progression of a cell into the M (mitosis) stage of the cell cycle.
Ensures that chromosomes are properly lined up prior to starting the next phase, anaphase.
A life stage that exists outside of the cell cycle. Cells can enter this phase due to poor environmental conditions, terminal differentiation, or other cellular cues.
“Undifferentiated or partially differentiated cells that can differentiate into various types of cells and proliferate indefinitely to produce more of the same stem cell.” Source: Stem cell. (2023, July 17). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Stem_cell
A microtubule arrangement established during mitosis.
An invisible axis where chromosomes line up during metaphase.
A protein kinase that, when activated, phosphorylates target proteins needed for progression to the next stage of the cell cycle. Activation requires binding with a cyclin protein as well as phosphorylation of particular locations within the protein.
An enzyme that adds a phosphate chemical group to another enzyme. These can be added on serines, threonines, or tyrosine.
“A family of proteins that controls the progression of a cell through the cell cycle by activating cyclin-dependent kinase (CDK) enzymes or group of enzymes required for synthesis of cell cycle.” Source: Cyclin. (2023, January 29). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Cyclin
A complex of a cyclin protein with a cyclin-dependent kinase (CDK). When this complex is activated, it promotes progression to the next stage of the cell cycle.
A regulated process of cell death.
A form of mutation that causes a gene to produce altered products that have gained new molecular functions compared to the unaltered gene products.
A form of mutation that causes a gene to produce proteins that are lacking molecular functions present in the nonmutated protein.
A region at the end of linear chromosomes with repetitive sequences. Specialized proteins will bind to this area. In addition, these repetitive sequences contain no genes and protect the genetic loss when the chromosomes are repeatedly replicated.
A structural region of a chromosome where the two sister chromatids are connected. Additionally, the kinetochore and mitotic spindle fibers attach to the sister chromatids during mitosis and meiosis.
Identical copies of a chromatid that are attached at the centromere after genome duplication in S phase. They will remain attached until anaphase, when they are pulled apart toward opposite spindle poles. Each sister chromatid represents one half of a replicated chromosome.
A protein complex that helps keep sister chromatids attached after DNA replication and also helps organize the interphase genome.
Specific sites on DNA where cohesins bind to attach sister chromatids together.
Proteins that bind to DNA to package into nucleosomes. They are highly basic, resulting in a positive charge. This allows them to bind to the negative backbone of DNA nonselectively.
A family of proteins with a role in DNA condensation needed for mitosis and meiosis.
A type of intermediate filament found in animal cells. They polymerize to form the nuclear lamina.
A membraneless organelle within the nucleus where ribosome biogenesis takes place.
Causes the degradation of the cohesion proteins that bind sister chromatids together, which releases the daughter chromosomes so that they can be pulled toward their respective spindle pole.
“A disc-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers attach during cell division to pull sister chromatids apart.” Source: Kinetochore. (2023, March 23). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Kinetochore
A regulatory mechanism whereby the products of a process will positively influence (upregulate) themselves.
A regulatory mechanism whereby the product of a process will negatively influence (downregulate) itself.
“A small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms.” Source: Ubiquitin. (2023, July 18). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Ubiquitin
“In this process, a sample containing cells or particles is suspended in a fluid and injected into the flow cytometer instrument. The sample is focused to ideally flow one cell at a time through a laser beam, where the light scattered is characteristic to the cells and their components. Cells are often labeled with fluorescent markers so light is absorbed and then emitted in a band of wavelengths. Tens of thousands of cells can be quickly examined and the data gathered are processed by a computer.” Source: Flow cytometry. (2023, June 27). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Flow_cytometry
Mutants of a gene where the result is a lack of activity when exposed to its restrictive temperatures. Often proper function is restored when brought back to the optimal (permissive) temperature.
A machine that analyzes properties (such as fluorescence) as it passes through.
A molecular motor that uses microtubules. Most kinesin motors move toward the plus end, but not all.
The first phase of mitosis. The DNA begins to condense and the mitotic spindle begins to form.
The microtubules emanating from a spindle pole that attach in the center (between the two poles).
A microtubule emanating from the spindle pole that attaches to the kinetochore of a chromosome during mitosis.
Microtubules that start at the spindle pole but extend toward the plasma membrane to anchor the spindle pole.
A molecular motor that uses microtubules. All known dynein motors move toward the minus end. Plants do not have dyneins and instead use minus-end directed kinesin to fulfill this role.
The intermediary mitotic phase between prophase and metaphase. Distinct in this phase is nuclear lamina breakdown and further attachment of kinetochore microtubules.
The stage of mitosis where chromosomes are lined up in a line on the metaphase plate.
A specialized tubulin network that acts as a nucleating site for microtubule growth.
In this part of Anaphase, the kinetochore microtubules shorten to facilitate chromosomes moving closer to their respective spindle poles.
In this part of Anaphase, the interpolar microtubules lengthen. This pushes the spindle poles further apart.
The last stage of mitosis, characterized by the DNA decondensing and the nuclear envelope reforming.
Fibrous mesh formed by intermediate filaments and membrane-associated proteins found just inside the nuclear envelope of cells. It functions as a structural support and helps maintain chromosome organization among other functions.
A special formation of actin filaments and myosin motors that will continually rachet smaller. This structure is needed for cytokinesis in animal cells.
“A plant cell specific structure that forms during late cytokinesis. It serves as a scaffold for cell plate assembly and subsequent formation of a new cell wall separating the two daughter cells.” Source: Phragmoplast. (2023, May 12). In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Phragmoplast
The site at which a new cell wall forms when a plant cell undergoes cytokinesis.
When cell division does not equally split a cell in half. This occurs in a variety of cell types.

