3 Surveillance
Kelly Johnson and Marit L. Bovbjerg
Learning Objectives
- Define epidemic and explain that word’s relationship to epidemiology
- Define surveillance and explain how surveillance relates to epidemiology overall
- Describe some common surveillance systems and methods used in the US
- Explain the rationale behind notifiable condition reporting, and how this pertains to epidemics, epidemiology, and surveillance
The root word for epidemiology is epidemic. An epidemic is “an increase, often sudden, in the number of cases of disease above what is normally expected in that population in that area.”i
How do we know how much is “expected”? Surveillance!
Public health surveillance is defined by the World Health Organization (WHO) as
the continuous, systematic collection, analysis, and interpretation of health related data needed for the planning, implementation, and evaluation of public health practice. Such surveillance can (1) serve as an early warning system for impending public health emergencies, (2) document the impact of an intervention, or track progress towards specified goals, and (3) monitor and clarify the epidemiology of health problems, to allow priorities to be set and to inform public health policy and strategies.ii
Surveillance activities can be either passive or active. In passive surveillance, the health department passively receives reports of suspected injury or illness. Think of this as waiting for disease reports to come to you. Many routine surveillance activities are passive—for instance, systems keeping track of communicable diseases, cancer, and injuries. Epidemiologists collect case reports that are sent to them by health care providers, laboratories, schools, or other entities that are required by law to report this information. In active surveillance, on the other hand, epidemiologists actively seek out cases of disease. For example, during an outbreak of salmonellosis associated with a specific source (say, a restaurant), epidemiologists may contact health care providers in the area and ask each for a list of patients seen with symptoms consistent with salmonellosis. These patients are then contacted to see if they were exposed to the suspected source (here, the restaurant). National surveys, such as the National Health and Nutrition Examination Survey (NHANES),iii are also considered active surveillance. The benefit of active surveillance is that it generally results in more complete data, while passive surveillance relies on others (who have numerous duties other than disease reporting) to report cases. The downside to active surveillance is that it is more resource-intensive, with increased personnel and financial requirements.iv
Some surveillance activities can be further characterized as population-based. The goal of population-based surveillance is to find every case that occurs within a population, and it is usually part of a mandated effort to collect cases of a specific condition of interest. An example of a condition for which we do population-based surveillance is cancer. Cancer registries aim to capture every case of cancer that occurs in the population the registry covers. This allows clinicians and public health professionals to monitor for trends in diagnoses that might signify a concerning change in the environment and/or for trends in survival that might follow improvements in treatment.
When reporting a specific condition is not required by law, public health officials must estimate incidence and prevalence in other ways. Sentinel surveillance involves case reporting from a limited number of hand-picked reporting sites. This type of surveillance is conducted when high-quality data are needed, passive systems are unable to provide these data, and resources are too scarce for complete, population-based active surveillance.v For example, annual influenza virus surveillance collects positive influenza specimens from a variety of selected sites each year for genotyping. From these specimens, we are able to determine which strains of the influenza virus are circulating each year and thus which strains to include in the annual influenza vaccine. Surveys can also be used to conduct surveillance on a representative sample of the population.
Notifiable Conditions
There is a list of conditions—mostly infectious diseases, but a few chronic diseases and injuries also make the list—that must be reported to the Centers for Disease Control and Prevention (CDC) whenever they are encountered by clinicians or health department officials. For example, say a patient presents to a primary care clinic complaining of high fever, cough, and watery eyes followed by a full-body rash. The nurse practitioner who sees the patient diagnoses measles. This clinic must then report the measles case to the local health department, who in turn reports it to the state health department, who in turn reports it to the CDC. This reporting ideally happens quickly, in a matter of days (or within hours for a potentially major threat).
The list of nationally notifiable conditions is reviewed every year or so and revised according to current public health threats and priorities. For instance, Zika virus and its associated congenital conditions were added to the list in 2016. (The 2020 list of notifiable conditions can be found here.)
Some of the conditions on the list are extremely rare (human rabies, plague) or have even been eradicated (small pox). However, they remain on the notifiable conditions list because in these cases, our expected level (also called the endemic level) is 0, and these conditions are dangerous enough that even one suspected case would be cause for an immediate public health intervention.
Each condition on the list has an associated set of case criteria, so after available evidence for a given patient (including laboratory data, symptoms, relevant exposures, and physician diagnoses) is collected, it is compared against the case criteria to either confirm or rule out a given case report. These case criteria are in place to make sure that all epidemiologists are evaluating case reports consistently. For example, the current case criteria for Lyme disease, last revised in 2017, can be found here.
For most of the conditions, the reporting criteria specify that these are new cases so that incidence can be calculated from these data. However, there are exceptions to this—hepatitis C is challenging to identify in its initial stage because few patients exhibit symptoms,vi resulting in a large number of hepatitis C infections that are identified during laboratory testing for something unrelated or once symptoms of liver damage occur. For conditions like this, the CDC requests notification of any newly diagnosed cases—regardless of whether they are also a new onset case.
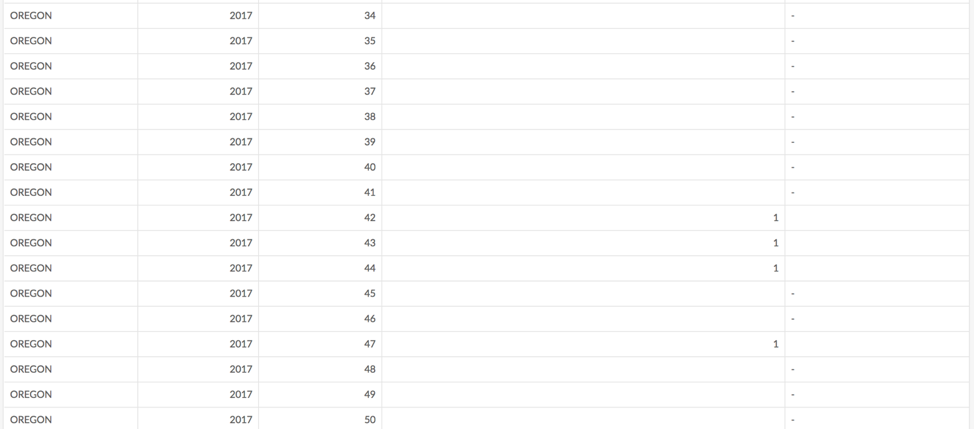
The CDC publishes weekly data tabulating all reported cases of the notifiable conditions.[1] Figure 3-1 shows a screenshot of the notifiable conditions tables for meningitis, in Oregon, during the last few months of 2017.vii
These cases were part of the epidemic of meningococcal meningitis that occurred at Oregon State University (OSU) during the 2017/2018 academic year—the expected number of cases of meningitis is 0, and the university, after consultation with the local and state health departments, took action (requiring students age 25 and younger to be vaccinated before they could register for classesviii) after only 6 cases were reported over the course of several weeks. The complete data tables for the notifiable conditions can be found here.
Notifiable Conditions and Privacy
At this point, most people are familiar with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule (45 CFR 154.512[b]). This rule states that your health care provider cannot disclose details about your health or the care that you received (collectively called protected health information) without your permission, with some exceptions for insurance and payments, coordinating care with other providers, and so on. Indeed, most clinics require that you acknowledge annually that they have informed you of their HIPAA-compliant privacy policies. Many people are unaware, however, that public health functions such as notifiable condition reporting are exempt from the HIPAA Privacy Rule. Indeed, the US Department of Health and Human Services states on its website, “The HIPAA Privacy Rule recognizes the legitimate need for public health authorities and others responsible for ensuring public health and safety to have access to protected health information to carry out their public health mission…Accordingly, the Rule permits covered entities to disclose protected health information without authorization for specified public health purposes.”
Source: https://www.hhs.gov/hipaa/for-professionals/special-topics/public-health/index.html
Cancer Registries
Cancer is a notifiable condition and is worth its own mention, because generally cancer reporting requirements are more extensive than those for other conditions. Depending on the state, a physician who diagnoses a type of cancer (other than non-melanoma skin cancers) must report extensive information to the health department, potentially including the type of tumor, the stage at which it was diagnosed, histology information, treatments given, and the eventual outcome (death, recurrence, etc.). Since cancer cases are reported upon diagnosis,[2] this can also be a potential source of incidence data, with the same caveats discussed above for hepatitis C (i.e., that sometimes a diagnosis occurs quite late in the disease process). Cancer registries are somewhat unique compared to other notifiable conditions data because patients are followed over time. Several states contribute their cancer registry data to the Surveillance, Epidemiology, and End Results (SEER) database,ix which is available for both surveillance and research purposes.
Vital Statistics
Birth and death certificates—together called vital statisticsx —constitute another ongoing surveillance system. Local hospitals report births and deaths up to their state health departments, who in turn report to the CDC. By keeping track of the health of newborns and childbearing women, as well as causes of death for everyone, public health officials can spot potential emerging trends that would warrant intervention. Annual reports summarizing all births and deaths that occurred in the US, as well as any notable changes from previous years, can be found on the CDC’s website here.
Survey-Based Surveillance Systems
The US conducts numerous surveillance activities that involve direct data collection from individual residents, usually via questionnaires, although NHANES includes physical exam and laboratory data as well. Other examples of surveillance include the Behavioral Risk Factor Surveillance System (BRFSS),xi which is a telephone-based survey of adults, who are asked to self-report their health and health behaviors; and the Pregnancy Risk Assessment Monitoring System (PRAMS),xii which is a paper-based survey of women who have recently given birth, who report on health and health care utilization for themselves and their newborn(s). Data from these surveys are used by public health professionals to monitor trends over time—for instance, the map on seat belt use shown in chapter 1 was made using BRFSS data—but they contain only data from prevalent cases. On the plus side, the survey data are freely available to students and researchers, and numerous articles are published each year using these datasets.
Conclusions
Surveillance activities allow epidemiologists and other public health professionals to monitor the “usual” levels of disease in a population. The ultimate goal of surveillance is to notice potential public health threats early so that a proper response can be mounted before a public health crisis ensues. The US has numerous surveillance systems operating at any one time, and much of the benefit from these systems develops as data are compared over time.
References
i. Principles of epidemiology: Lesson 1—section 11. Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/ophss/csels/dsepd/ss1978/lesson1/section11.html. Accessed September 10, 2018. (↵ Return)
ii. Public health surveillance. World Health Organization (WHO). http://www.who.int/topics/public_health_surveillance/en/. Accessed September 10, 2018. (↵ Return)
iii. National Health and Nutrition Examination Survey Homepage. CDC. 2018. https://www.cdc.gov/nchs/nhanes/index.htm. Accessed October 19, 2018. (↵ Return)
iv. Groseclose S, Sullivan K, Gibbs N, Knowles C. Management of the surveillance information system and quality control data. In: Teutsch S, Churchill R, eds. Principles and Practice of Public Health Surveillance. Vol 2. New York: Oxford University Press; 2000:95-111. (↵ Return)
v. Sentinel surveillance. WHO. http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/sentinel/en/. Accessed September 21, 2018. (↵ Return)
vi. Hepatitis C questions and answers for the public. CDC. 2018. https://www.cdc.gov/hepatitis/hcv/cfaq.htm. Accessed September 21, 2018. (↵ Return)
vii. Calgary O. Meningococcal to pertussis: NNDSS—table II. CDC. https://data.cdc.gov/NNDSS/NNDSS-Table-II-Meningococcal-to-Pertussis/hatw-7gqy/data. Accessed September 10, 2018. (↵ Return)
viii. Meningococcal disease. Student Health Services. 2009. https://studenthealth.oregonstate.edu/infectious-diseases/meningococcal-disease. Accessed October 19, 2018. (↵ Return)
ix. Surveillance, epidemiology, and end results program. National Cancer Institute. https://seer.cancer.gov/. Accessed October 20, 2018. (↵ Return)
x. National Vital Statistics System homepage: NVSS. CDC. 2018. https://www.cdc.gov/nchs/nvss/index.htm. Accessed October 20, 2018. (↵ Return)
xi. BRFSS—Behavioral Risk Factor Surveillance System. CDC. http://www.cdc.gov/brfss/. Accessed March 3, 2015. (↵ Return)
xii. Pregnancy Risk Assessment Monitoring System—reproductive health. CDC. http://www.cdc.gov/prams/. Accessed September 5, 2014. (↵ Return)
- It is important to note that underreporting is inherent in all passive surveillance systems, and the notifiable condition data are no different. ↵
- Cancer registries also include cases identified through autopsies/death certificates that were not diagnosed while the person was alive. Since not everyone has an autopsy when they die, undiagnosed cancers among people who have died are underreported. ↵
The occurrence, in a community or region, of cases of an illness (or specific health-related behaviour or other health-related events) clearly in excess of normal expectancy. Epidemiologists and other public health professionals keep track of what levels are "expected" through surveillance.
Determining the genetic composition of an organism. In terms of influenza, scientists genotype circulating flu viruses each year so that a targeted vaccine can be prepared.
The amount of a disease usually found in a given area. Known through surveillance.
Refers to laboratory/pathology findings related to what tissues or cells look like under a microscope.